2-Thiouracil
2-Thiouracil is a specific molecule consisting of a sulfated uracil.
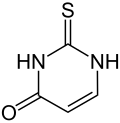 | |
| Names | |
|---|---|
| IUPAC name
2-Thioxo-1H-pyrimidin-4-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.008 |
| KEGG | |
| MeSH | Thiouracil |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H4N2OS | |
| Molar mass | 128.15 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Medical use
The substance is a historically relevant anti-thyroid preparation. Astwood E.B. used it in 1943 as therapy of Graves' disease for the first time.[1] It remains in use.
Thiouracil inhibits thyroid activity by blocking the enzyme thyroid peroxidase.[2] Its use in recent times has been replaced by advent of more potent and safer antithyroid drugs. It occurs in seeds of Brassica and Crucifera species. Thiouracil has been used as antithyroid, coronary vasodilator, and in congestive heart failure although its use has been largely supplanted by other drugs.[3]
References
- Gerabek, W. (2005). Enzyklopädie Medizingeschichte. p. 152. ISBN 978-3-11-015714-7.
- Nagasaka, A.; Hidaka, H. (1976). "Effect of Antithyroid Agents 6-Propyl-2-Thiouracil and l-Methyl-2-Mercaptoimidazole on Human Thyroid Iodide Peroxidase". Journal of Clinical Endocrinology & Metabolism. 43 (1): 152–8. doi:10.1210/jcem-43-1-152. PMID 947933.
- https://pubchem.ncbi.nlm.nih.gov/compound/thiouracil
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.