Acrylate
Acrylates (IUPAC: prop-2-enoates) are the salts, esters, and conjugate bases of acrylic acid. The acrylate ion is the anion CH2=CHCOO−. Often, acrylate refers to esters of acrylic acid, the most common member being methyl acrylate. These acrylates contain vinyl groups. These compounds are of interest because they are bifunctional: the vinyl group is susceptible to polymerization and the carboxylate group carries myriad functionalities. Modified acrylates are also numerous, some examples being methacrylates (CH2=C(CH3)CO2R) and cyanoacrylates (CH2=C(CN)CO2R).[1] Acrylate can also refer to polyacrylates prepared through the polymerization of the vinyl groups of acrylate monomers.[2]
- Structures of some acrylates and derivatives
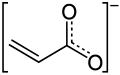 The acrylate anion
The acrylate anion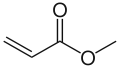 Methyl acrylate, an acrylic ester
Methyl acrylate, an acrylic ester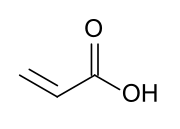
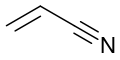
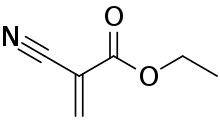 Ethyl cyanoacrylate, precursor to "super glue"
Ethyl cyanoacrylate, precursor to "super glue"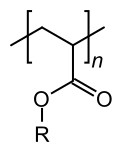 A generic polyacrylate (notice how the vinyl group has been functionalized)
A generic polyacrylate (notice how the vinyl group has been functionalized)
Use
Acrylates and methacrylates (the salts and esters of methacrylic acid) are common monomers in polymer plastics, forming the acrylate polymers. Acrylates easily form polymers. A variety of acrylate-functionalized monomers are known.[3]
Monomers
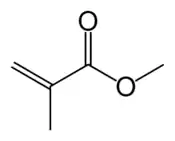
Acrylate monomers, used to form acrylate polymers, are based on the structure of acrylic acid,[4] which consists of a vinyl group and a carboxylic acid ester end or a nitrile.[5][6] Other typical acrylate monomers are derivatives of acrylic acid, such as methyl methacrylate in which one vinyl hydrogen and the carboxylic acid hydrogen are both replaced by methyl groups, and acrylonitrile in which the carboxylic acid group is replaced by the related nitrile group.
Other examples of acrylate monomers are:
- Methacrylates[7]
- Methyl acrylate
- Ethyl acrylate
- 2-Chloroethyl vinyl ether
- 2-Ethylhexyl acrylate
- Hydroxyethyl methacrylate
- Butyl acrylate
- Butyl methacrylate
- TMPTA
Production
Acrylates are industrially prepared by treating acrylic acid with the corresponding alcohol in presence of a catalyst. The reaction with lower alcohols (methanol, ethanol) takes place at 100–120 °C with acidic heterogeneous catalysts (cation exchanger). The reaction of higher alcohols (n-butanol, 2-ethylhexanol) is catalysed with sulfuric acid in homogeneous phase. Acrylates of even higher alcohols are obtainable by transesterification of lower esters catalysed by titanium alcoholates or organic tin compounds (e.g. dibutyltin dilaurate).[8]
See also
- Acrylate polymer
- Sodium polyacrylate thickeners
- Methacrylate
References
- Takashi Ohara; Takahisa Sato; Noboru Shimizu; Günter Prescher; Helmut Schwind; Otto Weiberg; Klaus Marten; Helmut Greim (2003). "Acrylic Acid and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_161.pub2.
- Veerle Coessens; Tomislav Pintauer; Krzysztof Matyjaszewski (2001). "Functional polymers by atom transfer radical polymerization". Progress in Polymer Science. doi:10.1016/S0079-6700(01)00003-X.
- Ervithayasuporn, Vuthichai; Chimjarn, Supansa (2013). "Synthesis and Isolation of Methacrylate- and Acrylate-Functionalized Polyhedral Oligomeric Silsesquioxanes (T8, T10, and T12) and Characterization of the Relationship between Their Chemical Structures and Physical Properties". Inorg. Chem. doi:10.1021/ic401994m.
- Mignon, Arn; Devisscher, Dries; Graulus, Geert-Jan; Stubbe, Birgit; Martins, José; Dubruel, Peter; De Belie, Nele; Van Vlierberghe, Sandra (2017-01-02). "Combinatory approach of methacrylated alginate and acid monomers for concrete applications". Carbohydrate Polymers. 155: 448–455. doi:10.1016/j.carbpol.2016.08.102. hdl:1942/22766. ISSN 0144-8617. PMID 27702534.
- Takashi Ohara; Takahisa Sato; Noboru Shimizu; et al. (2002). "Acrylic Acid and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_161.pub2. ISBN 978-3-527-30673-2.(subscription required)
- http://pslc.ws/macrog/acrylate.htm
- Manfred Stickler; Thoma Rhein (2000). "Polymethacrylates". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a21_473. ISBN 978-3-527-30673-2.(subscription required)
- Arpe, Hans-Jürgen (2007). Industrielle organische Chemie: bedeutende Vor- und Zwischenprodukte (6 ed.). Weinheim: Wiley-VCH. ISBN 978-3-527-31540-6.