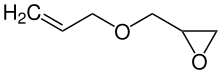
Allyl glycidyl ether
Allyl glycidyl ether is an organic compound used in adhesives and sealants and as a monomer for polymerization reactions. It is formally the condensation product of allyl alcohol and glycidol via an ether linkage. Because it contains both an alkene and an epoxide group, either group can be reacted selectively to yield a product where the other functional group remains intact for future reactions.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-{[(Prop-2-en-1-yl)oxy]methyl}oxirane | |
| Other names
2-[(Allyloxy)methyl]oxirane 1-Allyloxy-2,3-epoxypropane Glycidyl allyl ether [(2-Propenyloxy)methyl] oxirane[1] | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.003.131 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H10O2 | |
| Molar mass | 114.144 g·mol−1 |
| Appearance | Colorless liquid[1] |
| Odor | pleasant[1] |
| Density | 0.97 g/mL (20 °C)[1] |
| Melting point | −100 °C; −148 °F; 173 K [1] |
| Boiling point | 154 °C; 309 °F; 427 K [1] |
| 14% (20°C)[1] | |
| Solubility in organic solvents | miscible (acetone, toluene, octane)[2] |
| Vapor pressure | 2 mmHg (20 °C)[1] |
Refractive index (nD) |
1.4348 (20 °C)[2][3] |
| Hazards | |
| Main hazards | poisonous, mild irritant[2] |
| GHS Signal word | Danger |
| H226, H351, H341, H332, H302, H335, H315, H318, H317, H412 | |
| Flash point | 57 °C; 135 °F; 330 K [1] |
| Lethal dose or concentration (LD, LC): | |
LC50 (median concentration) |
270 ppm (mouse, 4 hr) 670 ppm (rat, 8 hr)[4] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
10 ppm (45 mg/m3)[1] |
REL (Recommended) |
TWA 5 ppm (22 mg/m3) ST 10 ppm (44 mg/m3) [skin][1] |
IDLH (Immediate danger) |
50 ppm[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
AGE is prepared commercially by the etherification of allyl alcohol with epichlorohydrin. Hydrogen chloride, the byproduct of their condensation, is removed with a base.[5]
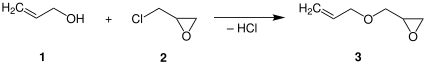
AGE can also be synthesized by monoepoxidation of diallyl ether.[6][7]
Diepoxidation of the second alkene would produce diglycidyl ether.
Allyl glycidyl ether is chiral. Most routes yield a racemic mixture. Epoxidation using monooxygenase enzyme proceeds enantioselectively.[8]

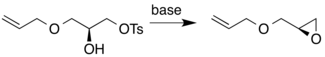
Alternately, nucleophilic cyclization of either chirality of the secondary alcohol onto a primary tosylate gives the chiral epoxide product.[9]
Uses
Allyl glycidyl ether is used in adhesives and sealants[2] and as a monomer for various types of polymer preparations.
Reactions
Polymerization
As a bifunctional compound, the alkene group or the epoxide group can be reacted selectively to yield a product where the other functional group remains intact for future reactions. For example, either one of them could be used for linear polymerization, and then the other used for cross-linking.[6]
Radical polymerization of the propylene portion in the presence of methyl acrylate yields a block copolymer with a high epoxide content.[10] Similarly, it is can be used in the production of polyvinylcaprolactam as a chain transfer agent.[11]
Nucleophilic polymerization of the epoxide groups gives a material that has the same backbone as polyethylene glycol, with allyl-ether side chains. The additional Lewis basic ether sites alter ion transport in the polymer and also affect the transient inter-chain crosslinking and glass transition temperature in the presence of metal ions. These properties suggest that the material may have applications as an alternative electrolyte for lithium-ion batteries. The alkenes can be elaborated into short polyethylene-glycol oligomers to further increase the ion-binding ability and enhance the resulting material properties.[12]
Block copolymers with ethylene oxide form micelles, which could be useful for encapsulating other molecules as part of a drug delivery system. The alkenes of these macromolecular structures can also be cross-linked via radical polymerization.[13]
Lewis-acid-catalyzed co-polymerisation with carbon dioxide likewise gives a polycarbonate material with allyl side chains that can be further elaborated.[14]
Hydrosilylation
Rather than polymerization, the alkene group can undergo a hydrosilylation reaction with siloxanes in the presence of chloroplatinic acid as catalyst.[15] Like the polymerization reactions, this reaction also leaves the epoxide intact. By this reaction, allyl glycidyl ether finds use as an intermediate in the production of silane coatings for electrical applications.[16]
References
- NIOSH Pocket Guide to Chemical Hazards. "#0019". National Institute for Occupational Safety and Health (NIOSH).
- CID 7838 from PubChem
- Clayton, G. D. and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981-1982., p. 2199
- "Allyl glycidyl ether". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Clayton, G. D.; Clayton, F. E., eds. (1981–1982). Patty's Industrial Hygiene and Toxicology. Volume 2A, 2B, 2C: Toxicology (3rd ed.). New York: John Wiley Sons. p. 2197.
- Frostick, Frederick C., Jr.; Phillips, Benjamin; Starcher, Paul S. (1959). "Synthesis of Some Epoxy Vinyl Monomers by Epoxidation with Peracetic Acid". J. Am. Chem. Soc. 81 (13): 3350–3356. doi:10.1021/ja01522a048.
- Wróblewska, Agnieszka; Drewnowska, E.; Gawarecka, A. (August 2016). "The epoxidation of diallyl ether to allyl-glycidyl ether over the TS-1 catalyst". Reaction Kinetics, Mechanisms and Catalysis. 118 (2): 719–931. doi:10.1007/s11144-016-1028-3.
- Fu, Hong; Newcomb, Martin; Wong, Chi Huey (1991). "Pseudomonas oleovorans monooxygenase-catalyzed asymmetric epoxidation of allyl alcohol derivatives and hydroxylation of a hypersensitive radical probe with the radical ring-opening rate exceeding the oxygen-rebound rate". J. Am. Chem. Soc. 113 (15): 5878–5880. doi:10.1021/ja00015a061.
- Pederson, Richard L.; Liu, Kevin K. C.; Rutan, James F.; Chen, Lihren; Wong, Chi Huey (1990). "Enzymes in organic synthesis: synthesis of highly enantiomerically pure 1,2-epoxy aldehydes, epoxy alcohols, thiirane, aziridine, and glyceraldehyde 3-phosphate". J. Org. Chem. 55 (16): 4897–4901. doi:10.1021/jo00303a026.
- Qingbo, Yu; Mingxu, Zhang; Xianhua, Li; Ruke, Bai (October 2007). "Living free-radical copolymerization of allyl glycidyl ether with methyl acrylate". Frontiers of Chemistry in China. 2 (4): 414–418. doi:10.1007/s11458-007-0078-5.
- Kudyshkin, Mukhitdinova (1999). "Control of the molecular weight of polyvinylcaprolactam". Russian Journal of Applied Chemistry. 72 (10): 1846–1848.
- Barteau, Katherine P.; Wolffs, Martin; Lynd, Nathaniel A.; Fredrickson, Glenn H.; Kramer, Edward J.; Hawker, Craig J. (2013). "Allyl Glycidyl Ether-Based Polymer Electrolytes for Room Temperature Lithium Batteries". Macromolecules. 46 (22): 8988–8994. Bibcode:2013MaMol..46.8988B. doi:10.1021/ma401267w.
- Hrubý, M.; Koňák, Č.; Ulbrich, K. (2004). "Poly(allyl glycidyl ether)‐block‐poly(ethylene oxide): A novel promising polymeric intermediate for the preparation of micellar drug delivery systems". Journal of Applied Polymer Science. 95 (2): 201–211. doi:10.1002/app.21121.
- Łukaszczyk, Jan; Jaszcz, Katarzyna; Kuran, Witold; Listos, Tomasz (2000). "Synthesis of functional polycarbonates by copolymerization of carbon dioxide with allyl glycidyl ether". Macromolecular Rapid Communications. 21 (11): 754–757. doi:10.1002/1521-3927(20000701)21:11<754::AID-MARC754>3.0.CO;2-O.
- "Allyl glycidyl ether". Sigma-Aldrich. Retrieved December 24, 2018.
- Ash, Michael; Ash, Irene, eds. (2007). Handbook of Fillers, Extenders, and Diluents. Synapse Info Resources. p. 224. ISBN 9781890595968.