Ameloblastoma
Ameloblastoma is a rare, benign tumor of odontogenic epithelium (ameloblasts, or outside portion, of the teeth during development) much more commonly appearing in the lower jaw than the upper jaw.[1] It was recognized in 1827 by Cusack.[2] This type of odontogenic neoplasm was designated as an adamantinoma in 1885 by the French physician Louis-Charles Malassez.[3] It was finally renamed to the modern name ameloblastoma in 1930 by Ivey and Churchill.[4][5]
| Ameloblastoma | |
|---|---|
 | |
| Micrograph of an ameloblastoma showing the characteristic palisading and stellate reticulum. H&E stain. | |
| Specialty | Oncology, oral and maxillofacial surgery |
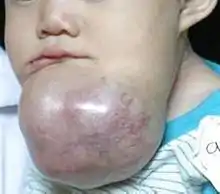
While these tumors are rarely malignant or metastatic (that is, they rarely spread to other parts of the body), and progress slowly, the resulting lesions can cause severe abnormalities of the face and jaw leading to severe disfiguration. Additionally, as abnormal cell growth easily infiltrates and destroys surrounding bony tissues, wide surgical excision is required to treat this disorder. If an aggressive tumor is left untreated, it can obstruct the nasal and oral airways making it impossible to breathe without oropharyngeal intervention. The term "ameloblastoma" is from the early English word amel, meaning enamel and the Greek word blastos, meaning germ.[6]
Types
Four types of ameloblastoma have been described by the WHO 2017 classification:[7]
- Conventional (solid/multicystic) type ameloblastoma
- Unicystic ameloblastoma
- Peripheral/extraosseous ameloblastoma
- Metastasising ameloblastoma
Conventional ameloblastoma
Previously known as solid/multicystic ameloblastoma. Usually presents with multiple large cystic areas.
Unicystic ameloblastoma
Ameloblastoma with a single cyst cavity account for around 10% of ameloblastomas. Present in younger patients in their second and third decades of life, often in relation to unerupted third molar.[8]
Metastasising ameloblastoma
Histologically atypical ameloblastoma can, rarely, lead to metastasis, usually in the lung. 'Metastasis' look histologically identical to the primary tumour and are benign in nature.[8]
Peripheral ameloblastoma
The peripheral subtype composes 2% of all ameloblastomas.[1]
Presentation
Ameloblastomas can be found both in the maxilla and mandible. Although, 80% are situated in the mandible with the posterior ramus area being the most frequent site.[9] The neoplasms are often associated with the presence of unerupted teeth, displacement of adjacent teeth and resorption of roots.[10]
Symptoms include a slow-growing, painless swelling leading to facial deformity. As the swelling gets progressively larger it can impinge on other structures resulting in loose teeth and malocclusion. Bone can also be perforated leading to soft tissue involvement.[9]
The lesion has a tendency to expand the bony cortices because the slow growth rate of the lesion allows time for the periosteum to develop a thin shell of bone ahead of the expanding lesion. This shell of bone cracks when palpated. This phenomenon is referred to as "Egg Shell Cracking" or crepitus, an important diagnostic feature.
Maxillary ameloblastomas can be dangerous and even lethal. Due to thin bone and weak barriers, the neoplasm can extend into the sinonasal passages, pterygomaxillary fossa and eventually into the cranium and brain.[8] Rare orbital invasion of the neoplasm has also been reported.[11]
Histopathology
Conventional ameloblastomas have both cystic and solid neoplastic structures.
Solid structure
Solid areas contain fibrous tissue islands or epithelium that interconnect through strands and sheets. The epithelial cells tend to move the nucleus away from the basement membrane to the opposite pole of the cell. This process is called reverse polarization. Two main histological patterns most often occur: follicular and plexiform. Other less common histological variants include acanthomatous, basal cell, and granular cell patterns.[8]
Follicular
The most common follicular type has an outer arrangement of columnar or palisaded ameloblasts-like cells and inner zone of triangular shaped cells resembling stellate reticulum from the bell stage of tooth development.[8]
Plexiform
The plexiform type has epithelium that proliferates in a "Fish Net Pattern". The plexiform ameloblastoma shows epithelium proliferating in a 'cord like fashion', hence the name 'plexiform'. There are layers of cells in between the proliferating epithelium with well-formed desmosomal junctions, simulating spindle cell layers. The ameloblasts cells can be less prominent.[8]
Cystic structure
Large cysts up to a few centimetres in diameter can be found. In follicular type, cysts develop in the stellate reticulum and in the plexiform type, cysts are caused by degeneration of connective tissue stroma.[8]
Desmoplastic ameloblastoma
A distinctive histological variant of conventional ameloblastoma. Found in near equal frequencies in both maxilla and mandible. Resemble a fibro-osseous lesion with no obvious ameloblasts whilst dominated by dense collagenous tissue (desmoplastic).[8] In one center, desmoplastic ameloblastomas represented about 9% of all ameloblastomas encountered.[12] A systematic review showed a predilection for males and predominance in fourth and fifth decades in life. 52% desmoplastic ameloblastomas showed mandibular involvement, with a tendency to anterior region. Majority of tumours were found to have ill-defined margins radiographically.[13]

Diagnosis
Ameloblastoma is tentatively diagnosed through radiographic examination and must be confirmed by histological examination through biopsy.[8]
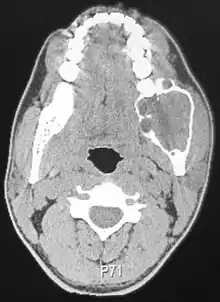
Radiographically, the tumour area appears as a rounded and well-defined lucency in the bone with varying size and features. Numerous cyst-like radiolucent areas can be seen in larger tumours (multi-locular) giving a characteristic "soap bubble" appearance. A single radiolucent area can be seen in smaller tumours (unilocular).[8] The radiodensity of an ameloblastoma is about 30 Hounsfield units, which is about the same as keratocystic odontogenic tumours. However, ameloblastomas show more bone expansion and seldom show high density areas.[14]
Lingual plate expansion is helpful in diagnosing ameloblastoma as cysts rarely do this. Resorption of roots of involved teeth can be seen in some cases, but is not unique to ameloblastoma.[10]
Differential diagnosis
Treatment
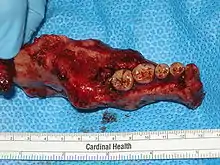
While chemotherapy, radiation therapy, curettage and liquid nitrogen have been effective in some cases of ameloblastoma, surgical resection or enucleation remains the most definitive treatment for this condition. However, in a detailed study of 345 patients, chemotherapy and radiation therapy was contraindicated for the treatment of ameloblastomas.[1] Thus, surgery is the most common treatment of this neoplasm. Conservative treatment requires very careful case selection.[8]
Surgical resection
The aim of treatment and surgery is to remove the entire tumour with a margin of surrounding tissue (block resection) for a good prognosis.[10] Preferable removal includes 10mm of normal bone around the neoplasm. Larger ameloblastomas can require partial resection of the jaw bone followed by bone grafting.[8] There is evidence that the treatment of conventional ameloblastoma is best done by bone resection.[15] A systematic review found that 79% of desmoplastic ameloblastoma cases were treated by resection.[13]
Enucleation
Smaller mandibular neoplasms have been enucleated where the cavity of the tumour is curetted, allowing preservation of the bone cortex and the lower border of the mandible. Although, recurrence rate for this type of treatment is higher. Unicystic ameloblastomas—called intraluminal unicystic or plexiform unicystic ameloblstomas can be enucleated, as the epithelium is only limited to the inner cyst wall and lumen.[8]
Radiation and chemotherapy
Radiation is ineffective in many cases of ameloblastoma.[1] There have also been reports of sarcoma being induced as the result of using radiation to treat ameloblastoma.[16] Chemotherapy is also often ineffective.[16] However, there is some controversy regarding this[17] and some indication that some ameloblastomas might be more responsive to radiation that previously thought.[18][5]
Follow-up and recurrence
Persistent follow-up examination including radiographs is essential for managing ameloblastoma.[19][8] Follow-up should occur at regular intervals for at least 10 years.[20] Follow up is important, because 50% of all recurrences occur within 5 years postoperatively.[1]
Recurrence is common, although the recurrence rates for block resection followed by bone graft are lower than those of enucleation and curettage.[21] Follicular variants appear to recur more than plexiform variants.[1] Unicystic lesions recur less frequently than "non-unicystic" lesions.[1] A low recurrence rate of around 10% can be seen in unicystic ameloblastomas.[8] Recurrence within a bone graft (following resection of the original tumor) does occur, but is less common.[22] Seeding to the bone graft is suspected as a cause of recurrence.[19] The recurrences in these cases seem to stem from the soft tissues, especially the adjacent periosteum.[23] Recurrence has been reported to occur as many as 36 years after treatment.[24] To reduce the likelihood of recurrence within grafted bone, meticulous surgery[22] with attention to the adjacent soft tissues is required.[23][19]
Molecular biology
BRAF V600E gene and SMO gene mutations have been found in ameloblastomas. V600E mutation is also seen in other malignant and benign neoplasms, which activate the MAP kinase pathway required for cell division and differentiation but is the most commonly seen mutation in ameloblastoma.[8][7] 72% of BRAF mutations are found in the mandible.[7] A recent study discovered a high frequency of BRAF V600E mutations (15 of 24 samples, 63%) in conventional ameloblastoma. These data suggests drugs targeting mutant BRAF as potential novel therapies for ameloblastoma.[25]
SMO mutations lead to the activation of the hedgehog pathway giving similar results as V600E but is less frequently seen.[8] 55% of SMO mutations are found in the maxilla.[7]
Evidence shows that suppression of matrix metalloproteinase-2 may inhibit the local invasiveness of ameloblastoma, however, this was only demonstrated in vitro.[26] There is also some research suggesting that α5β1 integrin may participate in the local invasiveness of ameloblastomas.[27]
Epidemiology
People with African heritage have been shown to have a higher incidence compared to Caucasians, with the site often being in the midline of the mandible.[10] The annual incidence rates per million for ameloblastomas are 1.96, 1.20, 0.18 and 0.44 for black males, black females, white males and white females respectively.[28] Ameloblastomas account for about one percent of all oral tumors[16] and about 18% of odontogenic tumors.[29] Men and women are equally affected, though women average four years younger than men when tumors first occur, and tumors run larger in females.[1]
See also
- Ameloblastic fibroma
- Bone grafting
- Epithelial cell rests of Malassez
- List of cutaneous conditions
- Matrix Metalloproteinase-2
- Tooth development and Odontogenesis
References
- Reichart PA, Philipsen HP, Sonner S (March 1995). "Ameloblastoma: biological profile of 3677 cases". European Journal of Cancer, Part B. 31B (2): 86–99. doi:10.1016/0964-1955(94)00037-5. PMID 7633291.
- Cusack JW (1827). "Report of the amputations of the lower jaw". Dublin Hosp Rec. 4: 1–38.
- Malassez L (1885). "Sur Le role des debris epitheliaux papdentaires". Arch Physiol Norm Pathol. 5: 309–340 6:379–449.
- Ivey RH, Churchill HR (1930). "The need of a standardized surgical and pathological classification of tumors and anomalies of dental origin". Am Assoc Dent Sch Trans. 7: 240–245.
- Madhup R, Kirti S, Bhatt ML, Srivastava M, Sudhir S, Srivastava AN (January 2006). "Giant ameloblastoma of jaw successfully treated by radiotherapy". Oral Oncology Extra. 42 (1): 22–25. doi:10.1016/j.ooe.2005.08.004.
- Brazis PW, Miller NR, Lee AG, Holliday MJ (1995). "Neuro-ophthalmologic Aspects of Ameloblastoma". Skull Base Surgery. 5 (4): 233–44. doi:10.1055/s-2008-1058921. PMC 1656531. PMID 17170964.
- Soluk-Tekkeşin M, Wright JM (2018). "The World Health Organization Classification of Odontogenic Lesions: A Summary of the Changes of the 2017 (4th) Edition". Turk Patoloji Dergisi. 34 (1). doi:10.5146/tjpath.2017.01410. PMID 28984343.
- W., Odell, E. (2017-06-28). Cawson's essentials of oral pathology and oral medicine. Preceded by (work): Cawson, R. A. (Ninth ed.). [Edinburgh]. ISBN 9780702049828. OCLC 960030340.
- Crispian S (2013). Oral and maxillofacial medicine : the basis of diagnosis and treatment (3rd ed.). Edinburgh: Churchill Livingstone/Elsevier. ISBN 9780702049484. OCLC 830037239.
- Coulthard P, Heasman PA (2008). Master dentistry (2nd ed.). Edinburgh: Churchill Livingstone/Elsevier. ISBN 9780702040047. OCLC 324993231.
- Abtahi MA, Zandi A, Razmjoo H, Ghaffari S, Abtahi SM, Jahanbani-Ardakani H, Kasaei Z, Kasaei-Koupaei S, Sajjadi S, Sonbolestan SA, Abtahi SH (March 2018). "Orbital invasion of ameloblastoma: A systematic review apropos of a rare entity". Journal of Current Ophthalmology. 30 (1): 23–34. doi:10.1016/j.joco.2017.09.001. PMC 5859465. PMID 29564405.
- Keszler A, Paparella ML, Dominguez FV (September 1996). "Desmoplastic and non-desmoplastic ameloblastoma: a comparative clinicopathological analysis". Oral Diseases. 2 (3): 228–31. doi:10.1111/j.1601-0825.1996.tb00229.x. PMID 9081764.
- Anand R, Sarode GS, Sarode SC, Reddy M, Unadkat HV, Mushtaq S, Deshmukh R, Choudhary S, Gupta N, Ganjre AP, Patil S (February 2018). "Clinicopathological characteristics of desmoplastic ameloblastoma: A systematic review". Journal of Investigative and Clinical Dentistry. 9 (1): e12282. doi:10.1111/jicd.12282. PMID 28707772.
- Ariji Y, Morita M, Katsumata A, Sugita Y, Naitoh M, Goto M, Izumi M, Kise Y, Shimozato K, Kurita K, Maeda H, Ariji E (March 2011). "Imaging features contributing to the diagnosis of ameloblastomas and keratocystic odontogenic tumours: logistic regression analysis". Dento Maxillo Facial Radiology. 40 (3): 133–40. doi:10.1259/dmfr/24726112. PMC 3611454. PMID 21346078.
- Almeida RD, Andrade ES, Barbalho JC, Vajgel A, Vasconcelos BC (March 2016). "Recurrence rate following treatment for primary multicystic ameloblastoma: systematic review and meta-analysis". International Journal of Oral and Maxillofacial Surgery. 45 (3): 359–67. doi:10.1016/j.ijom.2015.12.016. PMID 26792147.
- Randall S, Zane MD (3 January 2009). "Maxillary Ameloblastoma". Archived from the original on 2008-07-06.
- Atkinson CH, Harwood AR, Cummings BJ (February 1984). "Ameloblastoma of the jaw. A reappraisal of the role of megavoltage irradiation". Cancer. 53 (4): 869–73. doi:10.1002/1097-0142(19840215)53:4<869::AID-CNCR2820530409>3.0.CO;2-V. PMID 6420036.
- Miyamoto CT, Brady LW, Markoe A, Salinger D (June 1991). "Ameloblastoma of the jaw. Treatment with radiation therapy and a case report". American Journal of Clinical Oncology. 14 (3): 225–30. doi:10.1097/00000421-199106000-00009. PMID 2031509.
- Choi YS, Asaumi J, Yanagi Y, Hisatomi M, Konouchi H, Kishi K (January 2006). "A case of recurrent ameloblastoma developing in an autogenous iliac bone graft 20 years after the initial treatment". Dento Maxillo Facial Radiology. 35 (1): 43–6. doi:10.1259/dmfr/13828255. PMID 16421264.
- Su T, Liu B, Zhao JH, Zhang WF, Zhao YF (February 2006). "[Ameloblastoma recurrence in the grafted iliac bone: report of three cases]". Shanghai Kou Qiang Yi Xue = Shanghai Journal of Stomatology. 15 (1): 109–11. PMID 16525625.
- Vasan NT (March 1995). "Recurrent ameloblastoma in an autogenous bone graft after 28 years: a case report". The New Zealand Dental Journal. 91 (403): 12–3. PMID 7746553.
- Dolan EA, Angelillo JC, Georgiade NG (April 1981). "Recurrent ameloblastoma in autogenous rib graft. Report of a case". Oral Surgery, Oral Medicine, and Oral Pathology. 51 (4): 357–60. doi:10.1016/0030-4220(81)90143-2. PMID 7015222.
- Martins WD, Fávaro DM (December 2004). "Recurrence of an ameloblastoma in an autogenous iliac bone graft". Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics. 98 (6): 657–9. doi:10.1016/j.tripleo.2004.04.020. PMID 15583536.
- Zachariades N (October 1988). "Recurrences of ameloblastoma in bone grafts. Report of 4 cases". International Journal of Oral and Maxillofacial Surgery. 17 (5): 316–8. doi:10.1016/S0901-5027(88)80011-0. PMID 3143780.
- Kurppa KJ, Catón J, Morgan PR, Ristimäki A, Ruhin B, Kellokoski J, Elenius K, Heikinheimo K (April 2014). "High frequency of BRAF V600E mutations in ameloblastoma". The Journal of Pathology. 232 (5): 492–8. doi:10.1002/path.4317. PMC 4255689. PMID 24374844.
- Wang A, Zhang B, Huang H, Zhang L, Zeng D, Tao Q, Wang J, Pan C (June 2008). "Suppression of local invasion of ameloblastoma by inhibition of matrix metalloproteinase-2 in vitro". BMC Cancer. 8 (182): 182. doi:10.1186/1471-2407-8-182. PMC 2443806. PMID 18588710.
- Souza Andrade ES, da Costa Miguel MC, Pinto LP, de Souza LB (June 2007). "Ameloblastoma and adenomatoid odontogenic tumor: the role of alpha2beta1, alpha3beta1, and alpha5beta1 integrins in local invasiveness and architectural characteristics". Annals of Diagnostic Pathology. 11 (3): 199–205. doi:10.1016/j.anndiagpath.2006.04.005. PMID 17498594.
- Shear M, Singh S (July 1978). "Age-standardized incidence rates of ameloblastoma and dentigerous cyst on the Witwatersrand, South Africa". Community Dentistry and Oral Epidemiology. 6 (4): 195–9. doi:10.1111/j.1600-0528.1978.tb01149.x. PMID 278703.
- Gordon J (2004). "Clinical Quiz: Painless Mass". Medscape. 3 (8).
External links
| Classification | |
|---|---|
| External resources |
| Wikimedia Commons has media related to Ameloblastoma. |