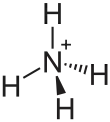
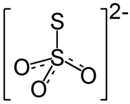
Ammonium thiosulfate
Ammonium thiosulfate (ammonium thiosulphate in British English) is an inorganic compound with the formula (NH4)2S2O3. It is white crystalline solid with ammonia odor, readily soluble in water, slightly soluble in acetone and insoluble in ethanol and diethyl ether.[1]
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Diammonium thiosulfate | |||
| Other names
Ammonium thiosulphate, ATS | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.029.074 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| H8N2O3S2 | |||
| Molar mass | 148.20 g·mol−1 | ||
| Appearance | colorless or white solid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Applications
Ammonium thiosulfate is used in photographic fixer. It is a so-called rapid fixer, acting more quickly than sodium thiosulfate fixers.[3] Fixation involves these chemical reactions (illustrated for silver bromide):[4]
- AgBr + 2 (NH4)2S2O3 → (NH4)3[Ag(S2O3)2] + NH4Br
- AgBr + 3 (NH4)2S2O3 → (NH4)5[Ag(S2O3)3] + NH4Br
Ammonium thiosulfate is also used for leaching of gold and silver. It works with presence of copper as a catalyst here. This process is a nontoxic alternative gold cyanidation.[5] The advantage to ammonium thiosulfate is that the pyrolysis of its silver complexes leaves a residue solely of silver sulfide, in contrast to complexes derived from sodium thiosulfate.[2]
References
- MSDS - Ammonium Thiosulfate
- J. J. Barbera; A. Metzger; M. Wolf (2012). "Sulfites, Thiosulfates, and Dithionites". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a25_477.
- Praní černobílých filmů a papírů
- Keller, Karlheinz (2005). "Photography". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a20_001.
- Aylmore, M.G; Muir, D.M (2001). "Thiosulfate leaching of gold—A review". Minerals Engineering. 14 (2): 135–174. doi:10.1016/S0892-6875(00)00172-2.
- McCarty, G. W.; Bremner1, J. M.; Krogmeier1, M. J. (1990). "Evaluation of ammonium thiosulfate as a soil urease inhibitor". Fertilizer Research. 24 (3): 135–139. doi:10.1007/BF01073581. S2CID 28574791.
- Wielgosiński, Grzegorz (2011). "The Reduction of Dioxin Emissions from the Processes of Heat and Power Generation". Journal of the Air & Waste Management Association. 61 (5): 511–526. doi:10.3155/1047-3289.61.5.511. PMID 21608491. S2CID 44546628.