Antibody-drug conjugate
Antibody-drug conjugates or ADCs are a class of biopharmaceutical drugs designed as a targeted therapy for treating cancer.[1] Unlike chemotherapy, ADCs are intended to target and kill tumor cells while sparing healthy cells. As of 2019, some 56 pharmaceutical companies were developing ADCs.[2]
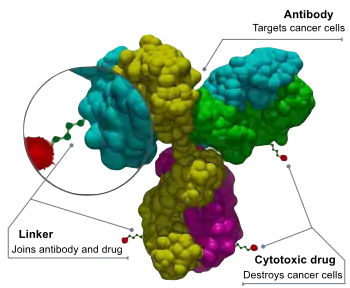
ADCs are complex molecules composed of an antibody linked to a biologically active cytotoxic (anticancer) payload or drug.[3] Antibody-drug conjugates are examples of bioconjugates and immunoconjugates.
ADCs combine the targeting capabilities of monoclonal antibodies with the cancer-killing ability of cytotoxic drugs. They can be designed to discriminate between healthy and diseased tissue.[4][5]
Mechanism of action
An anticancer drug is coupled to an antibody that specifically targets a certain tumor antigen (e.g. a protein that, ideally, is only to be found in or on tumor cells). Antibodies attach themselves to the antigens on the surface of cancerous cells. The biochemical reaction between the antibody and the target protein (antigen) triggers a signal in the tumor cell, which then absorbs or internalizes the antibody together with the linked cytotoxin. After the ADC is internalized, the cytotoxin kills the cancer.[6] This targeting, limits side effects and gives a wider therapeutic window than other chemotherapeutic agents.
ADC technologies have been featured in many publications,[7][8] including scientific journals.
History
Drugs that would target tumor cells and ignore others was conceived in 1900 by German Nobel laureate Paul Ehrlich.[2]
In 2001 Pfizer/Wyeth's drug Gemtuzumab ozogamicin (trade name: Mylotarg) was approved. However, after a request from the U.S. Food and Drug Administration (FDA), the company withdrew it in June 2010.[9] It was re-introduced into the US market in 2017.[10]
Brentuximab vedotin (trade name: Adcetris, marketed by Seattle Genetics and Millennium/Takeda)[11] was approved for relapsed HL and relapsed sALCL by the FDA on August 19, 2011 and received conditional marketing authorization from the European Medicines Agency in October 2012.
Trastuzumab emtansine (ado-trastuzumab emtansine or T-DM1, trade name: Kadcyla, marketed by Genentech and Roche) was approved in February 2013 for the treatment of people with HER2-positive metastatic breast cancer (mBC) who had received prior treatment with trastuzumab and a taxane chemotherapy.[12][13]
The European Commission approved Inotuzumab ozogamicin[14] as a monotherapy for the treatment of adults with relapsed or refractory CD22-positive B-cell precursor acute lymphoblastic leukemia (ALL) on June 30, 2017 under the trade name Besponsa® (Pfizer/Wyeth),[15] followed on August 17, 2017 by the FDA.[16]
The first immunology antibody-drug conjugate (iADC), ABBV-3373, is undergoing clinical trials for participants with moderate to severe rheumatoid arthritis.[17]
In July 2018, Daiichi Sankyo Company, Limited and Glycotope GmbH have inked a pact regarding the combination of Glycotope's investigational tumor-associated TA-MUC1 antibody gatipotuzumab and Daiichi Sankyo's proprietary ADC technology for developing gatipotuzumab antibody drug conjugate.[18]
In 2019 AstraZeneca agreed to pay up to US$6.9 billion to jointly develop DS-8201 with Japan's Daiichi Sankyo. It is intended to replace Herceptin for treating breast cancer. DS8201 carries eight payloads, compared to the usual four.[2]
Commercial products
Ten ADCs have received market approval – all for oncotherapies.
| Drug | Maker | Condition | Trade name | |
|---|---|---|---|---|
| Gemtuzumab ozogamicin | Pfizer/Wyeth | relapsed acute myelogenous leukemia (AML) | Mylotarg | |
| Brentuximab vedotin | Seattle Genetics, Millennium/Takeda | relapsed HL and relapsed sALCL | Adcetris | |
| Trastuzumab emtansine | Genentech, Roche | HER2-positive metastatic breast cancer (mBC) following treatment with trastuzumab and a maytansinoid | Kadcyla | |
| Inotuzumab ozogamicin | Pfizer/Wyeth | relapsed or refractory CD22-positive B-cell precursor acute lymphoblastic leukemia | Besponsa | |
| Polatuzumab vedotin-piiq | Genentech, Roche | relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL)[19] | Polivy | |
| Enfortumab vedotin | Astellas/Seattle Genetics | adult patients with locally advanced or metastatic urothelial cancer who have received a PD-1 or PD-L1 inhibitor, and a Pt-containing therapy[20] | Padcev | |
| Trastuzumab deruxtecan | AstraZeneca/Daiichi Sankyo | adult patients with unresectable or metastatic HER2-positive breast cancer who have received two or more prior anti-HER2 based regimens[21] | Enhertu | |
| Sacituzumab govitecan | Immunomedics | adult patients with metastatic triple-negative breast cancer (mTNBC) who have received at least two prior therapies for patients with relapsed or refractory metastatic disease[22] | Trodelvy | |
| Belantamab mafodotin | GlaxoSmithKline | multiple myeloma patients whose disease has progressed despite prior treatment with an immunomodulatory agent, proteasome inhibitor and anti-CD38 antibody[23] | Blenrep | |
| moxetumomab pasudotox-tdfk | AstraZeneca | relapsed or refractory (R/R) hairy cell leukemia (HCL) | Lumoxiti |
Linkers
A stable link between the antibody and cytotoxic (anti-cancer) agent is a crucial aspect of an ADC.[24] A stable ADC linker ensures that less of the cytotoxic payload falls off before reaching a tumor cell, improving safety, and limiting dosages.
Linkers are based on chemical motifs including disulfides, hydrazones or peptides (cleavable), or thioethers (noncleavable). Cleavable and noncleavable linkers were proved to be safe in preclinical and clinical trials. Brentuximab vedotin includes an enzyme-sensitive cleavable linker that delivers the antimicrotubule agent monomethyl auristatin E or MMAE, a synthetic antineoplastic agent, to human-specific CD30-positive malignant cells. MMAE inhibits cell division by blocking the polymerization of tubulin. Because of its high toxicity MMAE cannot be used as a single-agent chemotherapeutic drug. However, MMAE linked to an anti-CD30 monoclonal antibody (cAC10, a cell membrane protein of the tumor necrosis factor or TNF receptor) was stable in extracellular fluid. It is cleavable by cathepsin and safe for therapy. Trastuzumab emtansine is a combination of the microtubule-formation inhibitor mertansine (DM-1) and antibody trastuzumab that employs a stable, non-cleavable linker.
The availability of better and more stable linkers has changed the function of the chemical bond. The type of linker, cleavable or noncleavable, lends specific properties to the cytotoxic drug. For example, a non-cleavable linker keeps the drug within the cell. As a result, the entire antibody, linker and cytotoxic (anti-cancer) agent enter the targeted cancer cell where the antibody is degraded into an amino acid. The resulting complex – amino acid, linker and cytotoxic agent – is considered to be the active drug. In contrast, cleavable linkers are detached by enzymes in the cancer cell. The cytotoxic payload can then escape from the targeted cell and, in a process called "bystander killing", attack neighboring cells.[25]
Another type of cleavable linker, currently in development, adds an extra molecule between the cytotoxin and the cleavage site. This allows researchers to create ADCs with more flexibility without changing cleavage kinetics. Researchers are developing a new method of peptide cleavage based on Edman degradation, a method of sequencing amino acids in a peptide.[26] Also under development are site-specific conjugation (TDCs)[27] and novel conjugation techniques[28][29] to further improve stability and therapeutic index, α emitting immunoconjugates,[30] antibody-conjugated nanoparticles[31] and antibody-oligonucleotide conjugates.[32]
Research
Non-natural amino acids
The first generation uses linking technologies that conjugate drugs non-selectively to cysteine or lysine residues in the antibody, resulting in a heterogeneous mixture. This approach leads to suboptimal safety and efficacy and complicates optimization of the biological, physical and pharmacological properties.[27] Site-specific incorporation of unnatural amino acids generates a site for controlled and stable attachment. This enables the production of homogeneous ADCs with the antibody precisely linked to the drug and controlled ratios of antibody to drug, allowing the selection of a best-in-class ADC.[27] An Escherichia coli-based open cell-free synthesis (OCFS) allows the synthesis of proteins containing site-specifically incorporated non-natural amino acids and has been optimized for predictable high-yield protein synthesis and folding. The absence of a cell wall allows the addition of non-natural factors to the system to manipulate transcription, translation and folding to provide precise protein expression modulation.[33]
Other disease areas
The majority of ADCs under development or in clinical trials are for oncological and hematological indications.[34] This is primarily driven by the inventory of monoclonal antibodies, which target various types of cancer. However, some developers are looking to expand the application to other important disease areas.[35][36]
See also
References
- Hamilton GS (September 2015). "Antibody-drug conjugates for cancer therapy: The technological and regulatory challenges of developing drug-biologic hybrids". Biologicals. 43 (5): 318–32. doi:10.1016/j.biologicals.2015.05.006. PMID 26115630.
- Matsuyama K (2019-06-11). "Drug to replace chemotherapy may reshape cancer care". BNN Bloomberg. Retrieved 2019-06-14.
- Fitzpatrick-Dimond PF (March 9, 2010). "Antibody-Drug Conjugates Stage a Comeback". GEN: Genetic Engineering and Biotechnology News.
- DiJoseph JF, Armellino DC, Boghaert ER, Khandke K, Dougher MM, Sridharan L, et al. (March 2004). "Antibody-targeted chemotherapy with CMC-544: a CD22-targeted immunoconjugate of calicheamicin for the treatment of B-lymphoid malignancies". Blood. 103 (5): 1807–14. doi:10.1182/blood-2003-07-2466. PMID 14615373. S2CID 17543492.
- Mullard A (May 2013). "Maturing antibody-drug conjugate pipeline hits 30". Nature Reviews. Drug Discovery. 12 (5): 329–32. doi:10.1038/nrd4009. PMID 23629491. S2CID 28757488.
- Chari RV, Martell BA, Gross JL, Cook SB, Shah SA, Blättler WA, et al. (January 1992). "Immunoconjugates containing novel maytansinoids: promising anticancer drugs". Cancer Research. 52 (1): 127–31. PMID 1727373.
- Pollack A (May 31, 2012). "A One-Two Punch". The New York Times.
- "Ferrying a Drug Into a Cancer Cell". The New York Times. June 3, 2012.
- "FDA: Pfizer Voluntarily Withdraws Cancer Treatment Mylotarg from U.S. Market". U.S. Food and Drug Administration. Archived from the original on 2017-01-18.
- "Approved Drugs > FDA Approves Gemtuzumab Ozogamicin for CD33-positive AML". fda.gov. Silver Spring, USA: U.S. Food and Drug Administration. 1 September 2017. Retrieved 6 September 2017.
- "Brentuximab vedotin (SGN35)]". ADC Review/Journal of Antibody-drug Conjugates.
- "FDA Approves Genentech's Kadcyla® (Ado-Trastuzumab Emtansine), the First Antibody-Drug Conjugate for Treating Her2-Positive Metastatic Breast Cancer". Genentech.
- "Ado-trastuzumab emtansine". U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute.
- "Inotuzumab ozogamicin (drug description)". ADC Review/Journal of Antibody-drug Conjugates.
- "BESPONSA® Approved in the EU for Adult Patients with Relapsed or Refractory B-cell Precursor Acute Lymphoblastic Leukemia".
- "U.S. FDA Approves Inotuzumab Ozogamicin for Treatment of Patients with R/R B-cell precursor Acute Lymphoblastic Leukemia". ADC Review/Journal of Antibody-drug Conjugates. August 17, 2017.
- Clinical trial number NCT03823391 for "A Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Efficacy of ABBV-3373 in Participants With Moderate to Severe Rheumatoid Arthritis" at ClinicalTrials.gov
- "Antibody Drug Conjugate Market Size, Share, Trends, Growth Analysis Report, Application Immunotherapy, Business Opportunity Industry, Future Trends Forecast - 2023 |". September 24, 2019.
- Commissioner, Office of the (2019-06-10). "FDA approves first chemoimmunotherapy regimen for patients with relapsed or refractory diffuse large B-cell lymphoma". FDA. Retrieved 2019-06-14.
- "FDA grants accelerated approval to enfortumab vedotin-ejfv for metastatic urothelial cancer". FDA. 2019-12-18. Retrieved 2020-01-03.
- "FDA approves new treatment option for patients with HER2-positive breast cancer who have progressed on available therapies". FDA. 2019-12-20. Retrieved 2020-01-03.
- "FDA Approves New Therapy for Triple Negative Breast Cancer That Has Spread, Not Responded to Other Treatments". FDA. 2020-04-22. Retrieved 2020-04-24.
- "FDA granted accelerated approval to belantamab mafodotin-blmf for multiple myeloma". FDA. 2020-08-06. Retrieved 2020-08-06.
- Beck A, Goetsch L, Dumontet C, Corvaïa N (May 2017). "Strategies and challenges for the next generation of antibody-drug conjugates". Nature Reviews. Drug Discovery. 16 (5): 315–337. doi:10.1038/nrd.2016.268. PMID 28303026. S2CID 22045270.
- Kovtun YV, Goldmacher VS (October 2007). "Cell killing by antibody-drug conjugates". Cancer Letters. 255 (2): 232–40. doi:10.1016/j.canlet.2007.04.010. PMID 17553616.
- Bąchor R, Kluczyk A, Stefanowicz P, Szewczuk Z (August 2013). "New method of peptide cleavage based on Edman degradation". Molecular Diversity. 17 (3): 605–11. doi:10.1007/s11030-013-9453-y. PMC 3713267. PMID 23690169.
- Axup JY, Bajjuri KM, Ritland M, Hutchins BM, Kim CH, Kazane SA, et al. (October 2012). "Synthesis of site-specific antibody-drug conjugates using unnatural amino acids". Proceedings of the National Academy of Sciences of the United States of America. 109 (40): 16101–6. Bibcode:2012PNAS..10916101A. doi:10.1073/pnas.1211023109. PMC 3479532. PMID 22988081.
- Lyon RP, Setter JR, Bovee TD, Doronina SO, Hunter JH, Anderson ME, et al. (October 2014). "Self-hydrolyzing maleimides improve the stability and pharmacological properties of antibody-drug conjugates". Nature Biotechnology. 32 (10): 1059–62. doi:10.1038/nbt.2968. PMID 25194818. S2CID 5415162.
- Kolodych S, Koniev O, Baatarkhuu Z, Bonnefoy JY, Debaene F, Cianférani S, et al. (February 2015). "CBTF: new amine-to-thiol coupling reagent for preparation of antibody conjugates with increased plasma stability". Bioconjugate Chemistry. 26 (2): 197–200. doi:10.1021/bc500610g. PMID 25614935.
- Wulbrand C, Seidl C, Gaertner FC, Bruchertseifer F, Morgenstern A, Essler M, Senekowitsch-Schmidtke R (2013). Multhoff G (ed.). "Alpha-particle emitting 213Bi-anti-EGFR immunoconjugates eradicate tumor cells independent of oxygenation". PLOS ONE. 8 (5): e64730. Bibcode:2013PLoSO...864730W. doi:10.1371/journal.pone.0064730. PMC 3665541. PMID 23724085.
- Cardoso MM, Peça IN, Roque AC (2012). "Antibody-conjugated nanoparticles for therapeutic applications". Current Medicinal Chemistry. 19 (19): 3103–27. doi:10.2174/092986712800784667. hdl:10362/20689. PMID 22612698. S2CID 38141058.
- Dovgan I, Koniev O, Kolodych S, Wagner A (October 2019). "Antibody-Oligonucleotide Conjugates as Therapeutic, Imaging, and Detection Agents". Bioconjugate Chemistry. 30 (10): 2483–2501. doi:10.1021/acs.bioconjchem.9b00306. PMID 31339691.
- Zawada JF, Yin G, Steiner AR, Yang J, Naresh A, Roy SM, et al. (July 2011). "Microscale to manufacturing scale-up of cell-free cytokine production--a new approach for shortening protein production development timelines". Biotechnology and Bioengineering. 108 (7): 1570–8. doi:10.1002/bit.23103. PMC 3128707. PMID 21337337.
- Flygare JA, Pillow TH, Aristoff P (January 2013). "Antibody-drug conjugates for the treatment of cancer". Chemical Biology & Drug Design. 81 (1): 113–21. doi:10.1111/cbdd.12085. PMID 23253133. S2CID 20523083.
- Lehar SM, Pillow T, Xu M, Staben L, Kajihara KK, Vandlen R, et al. (November 2015). "Novel antibody-antibiotic conjugate eliminates intracellular S. aureus". Nature. 527 (7578): 323–8. Bibcode:2015Natur.527..323L. doi:10.1038/nature16057. PMID 26536114. S2CID 205246581.
- "Ambrx Collaborates with Merck to Design and Develop Biologic Drug Conjugates". Archived from the original (Press Release) on 2013-01-07.