Bacillithiol
Bacillithiol (BSH or Cys-GlcN-mal) is a thiol compound found in Bacillus species.[1] It is likely involved in maintaining cellular redox balance and plays a role in microbial resistance to the antibiotic fosfomycin.
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C13H22N2O10S | |
| Molar mass | 398.39 g/mol |
| Density | 1.629 g/mL |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Structure
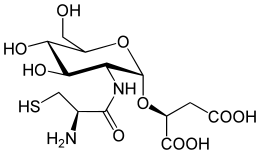
Chemically, it is a glycoside formed between L-cysteinyl-D-glucosamine and malic acid. It was isolated and identified (as its bacillithiol-S-bimane derivative) in 2009 from Staphylococcus aureus and Deinococcus radiodurans,[1] although it was first detected in 2007, as an unidentified thiol in Bacillus anthracis.[2] The naturally occurring free thiol form of bacillithiol has since been synthesised and characterised along with its biosynthetic precursors and its symmetrical disulfide.[3]
Biological role
Bacillithiol appears to participate in the sensing of peroxides by Bacillus,[4] but may also substitute for glutathione, which is the most common intracellular thiol in eukaryotes and some bacteria.[1] Some of the genes involved in the biosynthesis of bacillithiol were identified and characterized in 2010.[5] Bacteria engineered to be deficient in bacillithiol demonstrated increased sensitivity to various electrophilic xenobiotic compounds, including the antibiotic fosfomycin, suggesting that in these organisms the mechanism of fosfomycin resistance relies on the presence of bacillithiol.[5] Furthermore, in vitro kinetic studies have established that bacillithiol is a preferred thiol substrate for the antibiotic resistance enzyme FosB.[3][6]
Biosynthesis
Bacillithiol is produced via the enzymes BshA, BshB, and BshC. BshA replaces the UDP group on UDP-N-acetylglucosamine with an L-malyl group. BshB then removes the acetyl group. L-Cysteine is added to the resulting free amine, which completes the biosynthesis of the molecule. The cysteine-adding step is assumed to be carried out by the enzyme BshC on the basis of genetic knockout studies, but the activity of BshC has not been observed in vitro.[5][7]
See also
References
- Newton, G. L.; Rawat, M.; La Clair, J. J.; Jothivasan, V. K.; Budiarto, T.; Hamilton, C. J.; Claiborne, A.; Helmann, J. D.; Fahey, R. C. (2009). "Bacillithiol is an antioxidant thiol produced in Bacilli". Nature Chemical Biology. 5 (9): 625–627. doi:10.1038/nchembio.189. ISSN 1552-4450. PMC 3510479. PMID 19578333.
- Nicely, I.; Parsonage, D.; Paige, C.; Newton, L.; Fahey, C.; Leonardi, R.; Jackowski, S.; Mallett, C.; Claiborne, A. (Mar 2007). "STRUCTURE OF THE TYPE III PANTOTHENATE KINASE FROM Bacillus anthracis AT 2.0 Å RESOLUTION: IMPLICATIONS FOR COENZYME A-DEPENDENT REDOX BIOLOGY". Biochemistry. 46 (11): 3234–3245. doi:10.1021/bi062299p. ISSN 0006-2960. PMC 2613803. PMID 17323930.
- S. V. Sharma; V. K. Jothivasan; G. L. Newton; H. Upton; J. I.Wakabayashi; M. G. Kane; A. A. Roberts; M. Rawat; J. J. La Clair & C. J. Hamilton. (July 2011). "Chemical and Chemoenzymatic Syntheses of Bacillithiol: A Unique Low-Molecular-Weight Thiol amongst Low G + C Gram-Positive Bacteria". Angew. Chem. Int. Ed. 50 (31): 7101–7104. doi:10.1002/anie.201100196. PMID 21751306.
- Lee, W.; Soonsanga, S.; Helmann, D. (May 2007). "A complex thiolate switch regulates the Bacillus subtilis organic peroxide sensor OhrR". Proceedings of the National Academy of Sciences. 104 (21): 8743–8748. Bibcode:2007PNAS..104.8743L. doi:10.1073/pnas.0702081104. ISSN 0027-8424. PMC 1885573. PMID 17502599.
- Gaballa A, Newton GL, Antelmann H, et al. (April 2010). "Biosynthesis and functions of bacillithiol, a major low-molecular-weight thiol in Bacilli". Proc. Natl. Acad. Sci. U.S.A. 107 (14): 6482–6. Bibcode:2010PNAS..107.6482G. doi:10.1073/pnas.1000928107. PMC 2851989. PMID 20308541.
- A. A. Roberts; S. V. Sharma; A. W. Strankman; S. R. Duran; M. Rawat & C. J. Hamilton. (July 2013). "Mechanistic studies of FosB: a divalent-metal-dependent bacillithiol-S-transferase that mediates fosfomycin resistance in Staphylococcus aureus". Biochem. J. 451 (1): 69–79. doi:10.1042/BJ20121541. PMC 3960972. PMID 23256780.
- A. J. VanDuienen; K. R. Winchell & P. D. Cook (January 20, 2015). "X-ray Crystallographic Structure of BshC, a Unique Enzyme Involved in Bacillithiol Biosynthesis". Biochemistry. 541 (2): 100–103. doi:10.1021/bi501394q. PMC 4303302. PMID 25496067.
