Bismuth(III) nitrate
Bismuth(III) nitrate is a salt composed of bismuth in its cationic +3 oxidation state and nitrate anions. The most common solid form is the pentahydrate.[2] It is used in the synthesis of other bismuth compounds.[3] It is available commercially. It is the only nitrate salt formed by a group 15 element, indicative of bismuth's metallic nature.[4]
 | |
| Names | |
|---|---|
| Other names
Bismuth trinitrate, Bismuth(III) nitrate pentahydrate | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.030.707 |
| EC Number |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Bi(NO3)3·5H2O | |
| Molar mass | 485.07 g/mol (pentahydrate) |
| Appearance | colorless, white |
| Density | 2.90 g/cm3 (pentahydrate)[1] |
| -91.0·10−6 cm3/mol | |
| Hazards | |
| GHS pictograms |   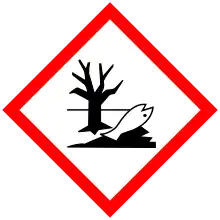 |
| GHS Signal word | Warning |
| H272, H315, H319, H335, H411 | |
| P210, P220, P221, P261, P264, P271, P273, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P370+378, P391, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation and reactions
Bismuth nitrate can be prepared by the reaction of bismuth metal and concentrated nitric acid.[5]
- Bi + 4HNO3 → Bi(NO3)3 + 2H2O + NO
It dissolves in nitric acid but is readily hydrolysed to form a range of oxynitrates when the pH increases above 0.[6]
It is also soluble in acetone, acetic acid and glycerol but practically insoluble in ethanol and ethyl acetate.[7]
Some uses in organic synthesis have been reported for example the nitration of aromatic compounds and selective oxidation of sulfides to sulfoxides.[7] It is also used as to form Dragendorff reagent, which is used as a TLC stain.
Bismuth nitrate forms insoluble complexes with pyrogallol and cupferron and these have been the basis of gravimetric methods of determining bismuth content.[8]
On heating bismuth nitrate can decompose forming nitrogen dioxide, NO2.[9]
Structure
The crystal form is triclinic, and contains 10 coordinate Bi3+, (three bidentate nitrate ions and four water molecules).[1]
References
- Lazarini, F. (15 August 1985). "Redetermination of the structure of bismuth(III) nitrate pentahydrate, Bi(NO3)3.5H2O". Acta Crystallographica Section C. 41 (8): 1144–1145. doi:10.1107/S0108270185006916.
- "Normal Bismuth Nitrate, Bi(NO3)3".
- Mary Eagleson (1994). Concise encyclopedia chemistry. Walter de Gruyter. ISBN 3-11-011451-8.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Rich, Ronald (2007). Inorganic Reactions in Water (e-book). Springer. ISBN 978-3-540-73962-3.
- Lazarini, F. (1981). "Thermal dehydration of some basic bismuth nitrates". Thermochimica Acta. 46 (1): 53–55. doi:10.1016/0040-6031(81)85076-9. ISSN 0040-6031.
- Suzuki, Hitomi, ed. (2001). Organobismuth Chemistry. Elsevier. ISBN 0-444-20528-4.
- A.I. Vogel,(1951), Quantitative Inorganic analysis, (2d edition), Longmans Green and Co
- Krabbe, S.W.; Mohan, R.S. (2012). "Environmentally friendly organic synthesis using Bi(III) compounds". In Ollevier, Thierry (ed.). Topics in Current chemistry 311, Bismuth-Mediated Organic Reactions. Springer. pp. 100–110. ISBN 978-3-642-27239-4.
| HNO3 | He | ||||||||||||||||
| LiNO3 | Be(NO3)2 | B(NO 3)− 4 |
RONO2 | NO− 3 NH4NO3 |
HOONO2 | FNO3 | Ne | ||||||||||
| NaNO3 | Mg(NO3)2 | Al(NO3)3 | Si | P | S | ClONO2 | Ar | ||||||||||
| KNO3 | Ca(NO3)2 | Sc(NO3)3 | Ti(NO3)4 | VO(NO3)3 | Cr(NO3)3 | Mn(NO3)2 | Fe(NO3)2 Fe(NO3)3 |
Co(NO3)2 Co(NO3)3 |
Ni(NO3)2 | CuNO3 Cu(NO3)2 |
Zn(NO3)2 | Ga(NO3)3 | Ge | As | Se | Br | Kr |
| RbNO3 | Sr(NO3)2 | Y(NO3)3 | Zr(NO3)4 | Nb | Mo | Tc | Ru(NO3)3 | Rh(NO3)3 | Pd(NO3)2 Pd(NO3)4 |
AgNO3 Ag(NO3)2 |
Cd(NO3)2 | In(NO3)3 | Sn | Sb(NO3)3 | Te | INO3 | Xe(NO3)2 |
| CsNO3 | Ba(NO3)2 | Hf(NO3)4 | Ta | W | Re | Os | Ir | Pt(NO3)2 Pt(NO3)4 |
Au(NO3)3 | Hg2(NO3)2 Hg(NO3)2 |
TlNO3 Tl(NO3)3 |
Pb(NO3)2 | Bi(NO3)3 BiO(NO3) |
Po(NO3)4 | At | Rn | |
| FrNO3 | Ra(NO3)2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La(NO3)3 | Ce(NO3)3 Ce(NO3)4 |
Pr(NO3)3 | Nd(NO3)3 | Pm(NO3)3 | Sm(NO3)3 | Eu(NO3)3 | Gd(NO3)3 | Tb(NO3)3 | Dy(NO3)3 | Ho(NO3)3 | Er(NO3)3 | Tm(NO3)3 | Yb(NO3)3 | Lu(NO3)3 | |||
| Ac(NO3)3 | Th(NO3)4 | PaO2(NO3)3 | UO2(NO3)2 | Np(NO3)4 | Pu(NO3)4 | Am(NO3)3 | Cm(NO3)3 | Bk | Cf | Es | Fm | Md | No | Lr | |||