Cell cycle regulated Methyltransferase
CcrM is an orphan DNA methyltransferase, that is involved in controlling gene expression in most Alphaproteobacteria. This enzyme modifies DNA by catalyzing the transference of a methyl group from the S-adenosyl-L methionine substrate to the N6 position of an adenine base in the sequence 5'-GANTC-3' with high specificity.[1] In Caulobacter crescentus Ccrm is produced at the end of the replication cycle when Ccrm recognition sites are hemimethylated, rapidly methylating the DNA. CcrM is essential in other Alphaproteobacteria but is role is not yet determined. CcrM is a highly specific methyltransferase with a novel DNA recognition mechanism.
CcrM role in cell cycle regulation
Methylations are epigenetic modification that, in eukaryotes, regulates processes as cell differentiation, and embryogenesis,[2] while in prokaryotes can have a role in self recognition, protecting the DNA from being cleaved by the restriction endonuclease system,[3] or for gene regulation. The first function is controlled by the restriction methylation system while the second by Orphan MTases as Dam and CcrM.[4]
CcrM role have been characterized in the marine model organism Caulobacter crescentus, which is suitable for the study of cell cycle and epigenetics as it asymmetrically divides generating different progeny, a stalked and a swarmer cell, with different phenotypes and gene regulation. The swarmer cell has a single flagellum and polar pili and is characterized by its mobility, while the stacked cell has a stalk and is fixed to the substrate. The stacked cell enters immediately in S-phase, while the swarmer cell stays in G1-phase and will differentiate to a stacked cell before entering the S-phase again.[5]
The stacked cell in S phase will replicate its DNA in a semiconservative manner producing two hemimethylated DNA double strands that will be rapidly methylated by the Methyltransferase CcrM, which is only produced at the end of the S phase. The enzyme will methylate more than 4 thousand 5'-GANTC-3' sites in around 20 minutes, and then it will be degraded by the LON protease.[6] This fast methylation plays an important role in the transcriptional control of several genes and controls the cell differentiation. CcrM expression is regulated by the CtrA master regulator, and in addition various 5'-GANTC-3' sites methylation sites regulate CcrM expression, which will only occur at the end of the S phase when this sites are hemimethylated. In this process CtrA regulates the expression of CcrM and more than 1000 genes in the pre-divisional state,[7] and SciP prevents the activation of CcrM transcription in non replicative cells.
CcrM role in Alphaproteobacteria
Orphan MTases are common in bacteria and archea[8] CcrM is found in almost every group of Alphaproteobacteria, excepting in Rickettsiales and Magnetococcales, and homologs can be found inEpsilonproteobacteria and Gammaproteobacteria.[9] Alphaproteobacteria are organisms with different life stages from free living to substrate associated, some of them are intracellular pathogens of plants, animal and even human,[10] in those groups the CcrMs must have an important role in cell cycle progression.[11]
CcrM miss regulation have shown to produce severe miss control of cell cycle regulation and differentiation in various Alphaproteobacteria; C. crescentus , the plant symbiont Sinorhizobium meliloti[12] and in the human pathogen Brucella abortus.[13] Also CcrM gene has proven to be essential for the viability of various Alphaproteobacteria.[10]
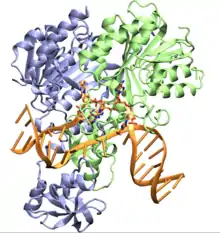
Structure and DNA recognition mechanism
CcrM is a type II DNA Methyltransferase, that transfer a methyl group from the methyl donor SAM to the N6 of an adenine in a 5'-GANTC-3' recognition sites of hemimethylated DNA. Based on the order of the conserved motifs that form the SAM binding, the active site and the target recognition domain in the sequence of CcrM it can be classified as a β-class adenine N6 Methyltransferase.[14] CcrM homologs in Alphaproteobacteria have an 80 residues C terminal domain,[9] with non well characterized function.[15]
CcrM is characterized by a high degree of sequence discrimination, showing a very high specificity for GANTC sites over AANTC sites , being able to recognize and methylate this sequence in both double and single strand DNA.[1] CcrM in complex with a dsDNA structure was resolved, showing that the enzyme presents a novel DNA interaction mechanism, opening a bubble in the DNA recognition site (The concerted mechanism of Methyltransferases relies in the flip of the target base), the enzyme interacts with DNA forming an homodimer with differential monomer interactions.[16]
CcrM is a highly efficient enzyme capable of methylating a high number of 5'-GANTC-3' sites in low time, however if the enzyme is processive (the enzyme binds to the DNA and methylate several methylation sites before dissociation) or distributive (the enzyme dissociates from DNA after each methylation) it is still in discussion. First reports indicated the second case,[17] however more recent characterisation of CcrM indicate that it is a processive enzyme.[18]
References
- Reich, Norbert O.; Dang, Eric; Kurnik, Martin; Pathuri, Sarath; Woodcock, Clayton B. (2018-12-07). "The highly specific, cell cycle–regulated methyltransferase from Caulobacter crescentus relies on a novel DNA recognition mechanism". Journal of Biological Chemistry. 293 (49): 19038–19046. doi:10.1074/jbc.RA118.005212. ISSN 0021-9258. PMC 6295719. PMID 30323065.
- Schübeler, Dirk (January 2015). "Function and information content of DNA methylation". Nature. 517 (7534): 321–326. doi:10.1038/nature14192. ISSN 1476-4687. PMID 25592537.
- Vasu, K.; Nagaraja, V. (2013-03-01). "Diverse Functions of Restriction-Modification Systems in Addition to Cellular Defense". Microbiology and Molecular Biology Reviews. 77 (1): 53–72. doi:10.1128/MMBR.00044-12. ISSN 1092-2172. PMC 3591985. PMID 23471617.
- Adhikari, Satish; Curtis, Patrick D. (2016-09-01). "DNA methyltransferases and epigenetic regulation in bacteria". FEMS Microbiology Reviews. 40 (5): 575–591. doi:10.1093/femsre/fuw023. ISSN 0168-6445. PMID 27476077.
- Laub, Michael T.; Shapiro, Lucy; McAdams, Harley H. (December 2007). "Systems Biology of Caulobacter". Annual Review of Genetics. 41 (1): 429–441. doi:10.1146/annurev.genet.41.110306.130346. ISSN 0066-4197. PMID 18076330.
- Collier, J.; McAdams, H. H.; Shapiro, L. (2007-10-23). "A DNA methylation ratchet governs progression through a bacterial cell cycle". Proceedings of the National Academy of Sciences. 104 (43): 17111–17116. doi:10.1073/pnas.0708112104. ISSN 0027-8424. PMC 2040471. PMID 17942674.
- Collier, Justine (April 2016). "Cell cycle control in Alphaproteobacteria". Current Opinion in Microbiology. 30: 107–113. doi:10.1016/j.mib.2016.01.010. PMID 26871482.
- Staff, The PLOS Genetics (2016-05-12). "Correction: The Epigenomic Landscape of Prokaryotes". PLOS Genetics. 12 (5): e1006064. doi:10.1371/journal.pgen.1006064. ISSN 1553-7404. PMC 4865105. PMID 27171000.
- Gonzalez, Diego; Kozdon, Jennifer B.; McAdams, Harley H.; Shapiro, Lucy; Collier, Justine (April 2014). "The functions of DNA methylation by CcrM in Caulobacter crescentus: a global approach". Nucleic Acids Research. 42 (6): 3720–3735. doi:10.1093/nar/gkt1352. ISSN 1362-4962. PMC 3973325. PMID 24398711.
- Mouammine, Annabelle; Collier, Justine (October 2018). "The impact of DNA methylation in Alphaproteobacteria". Molecular Microbiology. 110 (1): 1–10. doi:10.1111/mmi.14079. ISSN 0950-382X. PMID 29995343.
- Collier, Justine (December 2009). "Epigenetic regulation of the bacterial cell cycle". Current Opinion in Microbiology. 12 (6): 722–729. doi:10.1016/j.mib.2009.08.005. PMID 19783470.
- Wright, R; Stephens, C; Shapiro, L (September 1997). "The CcrM DNA methyltransferase is widespread in the alpha subdivision of proteobacteria, and its essential functions are conserved in Rhizobium meliloti and Caulobacter crescentus". Journal of Bacteriology. 179 (18): 5869–5877. doi:10.1128/jb.179.18.5869-5877.1997. ISSN 0021-9193. PMC 179479. PMID 9294447.
- Robertson, Gregory T.; Reisenauer, Ann; Wright, Rachel; Jensen, Rasmus B.; Jensen, Allen; Shapiro, Lucille; Roop, R. Martin (2000-06-15). "The Brucella abortus CcrM DNA Methyltransferase Is Essential for Viability, and Its Overexpression Attenuates Intracellular Replication in Murine Macrophages". Journal of Bacteriology. 182 (12): 3482–3489. doi:10.1128/JB.182.12.3482-3489.2000. ISSN 0021-9193. PMC 101938. PMID 10852881.
- Murphy, James; Mahony, Jennifer; Ainsworth, Stuart; Nauta, Arjen; van Sinderen, Douwe (2013-12-15). "Bacteriophage Orphan DNA Methyltransferases: Insights from Their Bacterial Origin, Function, and Occurrence". Applied and Environmental Microbiology. 79 (24): 7547–7555. doi:10.1128/AEM.02229-13. ISSN 0099-2240. PMC 3837797. PMID 24123737.
- Maier, Johannes A.H.; Albu, Razvan F.; Jurkowski, Tomasz P.; Jeltsch, Albert (December 2015). "Investigation of the C-terminal domain of the bacterial DNA-(adenine N6)-methyltransferase CcrM". Biochimie. 119: 60–67. doi:10.1016/j.biochi.2015.10.011. PMID 26475175.
- Horton, John R.; Woodcock, Clayton B.; Opot, Sifa B.; Reich, Norbert O.; Zhang, Xing; Cheng, Xiaodong (2019-10-10). "The cell cycle-regulated DNA adenine methyltransferase CcrM opens a bubble at its DNA recognition site". Nature Communications. 10 (1): 4600. doi:10.1038/s41467-019-12498-7. ISSN 2041-1723. PMC 6787082. PMID 31601797.
- Albu, R. F.; Jurkowski, T. P.; Jeltsch, A. (2012-02-01). "The Caulobacter crescentus DNA-(adenine-N6)-methyltransferase CcrM methylates DNA in a distributive manner". Nucleic Acids Research. 40 (4): 1708–1716. doi:10.1093/nar/gkr768. ISSN 0305-1048. PMC 3287173. PMID 21926159.
- Woodcock, Clayton B.; Yakubov, Aziz B.; Reich, Norbert O. (August 2017). "Caulobacter crescentus Cell Cycle-Regulated DNA Methyltransferase Uses a Novel Mechanism for Substrate Recognition". Biochemistry. 56 (30): 3913–3922. doi:10.1021/acs.biochem.7b00378. ISSN 0006-2960. PMID 28661661.