Chemical ionization
Chemical ionization (CI) is a soft ionization technique used in mass spectrometry.[1][2] This was first introduced by Burnaby Munson and Frank H. Field in 1966.[3] This technique is a branch of gaseous ion-molecule chemistry.[2] Reagent gas molecules are ionized by electron ionization, which subsequently react with analyte molecules in the gas phase in order to achieve ionization. Negative chemical ionization (NCI), charge-exchange chemical ionization and atmospheric-pressure chemical ionization (APCI) are some of the common variations of this technique. CI has several important applications in identification, structure elucidation and quantitation of organic compounds.[4] Beside the applications in analytical chemistry, the usefulness in chemical ionization extends toward biochemical, biological and medicinal fields as well.[4]
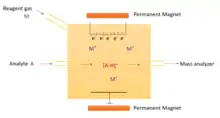
Principles of operation
Chemical ionization requires a lower amount of energy compared to electron ionization (EI), but this depends on the reactant material used.[2] This low-energy ionization mechanism yields less or sometimes no fragmentation, and usually a simpler spectrum. The lack of fragmentation limits the amount of structural information that can be determined about the ionized species. However, a typical CI spectrum has an easily identifiable protonated molecular ion peak [M+1]+, which allows easy determination of molecular mass.[5] This technique requires the transfer of high-mass entities from the reagent gas to the analyte, and therefore, the Franck-Condon principle does not govern the process of ionization. CI is thus quite useful in cases where the energy of the bombarding electrons in EI is high, resulting exclusively in fragmentation of the analyte, causing the molecular-ion peak to be less detectable or completely absent.
Instrumentation
CI uses a common source for ionization as EI, with some modifications. To facilitate the reactions between the ions-gases, the chamber is kept gastight with a pressure around 1 torr.[6] Electrons are produced through a metal filament, which is made of tungsten, rhenium, or iridium[4] and travel to a longer distance in the ionization chamber, due to high energy it owns.[6] In contrast to EI, the magnet and the electron trap is not needed for CI, since the electron beam do not travel to the end of the chamber. The pressure inside the chamber is kept below 10−4 torr.[6]
Mechanism
A CI experiment involves the use of gas phase acid-base reactions in the chamber. Ions are produced through the collision of the analyte with ions of a reagent gas that are present in the ion source. Some common reagent gases include: methane, ammonia, water and isobutane. Inside the ion source, the reagent gas is present in large excess compared to the analyte. Electrons entering the source with energy around 200-500 eV[6] will preferentially ionize the reagent gas. Then, the ion/molecule reactions produces more stable reagent ions and the resultant collisions with other reagent gas molecules will create an ionization plasma. Positive and negative ions of the analyte are formed by reactions with this plasma.[5]
The following reactions are possible with methane as the reagent gas.
Primary ion formation
Secondary reagent ions
Product ion formation
- (protonation)
- ( abstraction)
- (adduct formation)
If ammonia is the reagent gas,
For isobutane as the reagent gas,
Self chemical ionization is possible if the reagent ion is an ionized form of the analyte.[7]
Advantages and limitations
The high energy molecular ions produced by the bombardment with electrons pass their energy to neutral molecules via collision.[6] This allows the analytes to be less fragmented and therefore molecular weight of an unknown analyte can be determined. The extent of fragmentation is controlled by proper selection of reagent gases.[6] The spectra given by CI is simpler and more sensitive[4] compared to other ionization methods. Moreover, some variations of CI can be coupled to chromatographic separation techniques, thereby improving its usefulness in identification of compounds.[8] However, this method is restricted to volatile compounds and due to less fragmentation, lesser amount of information can be obtained.
Applications

CI mass spectrometry is a useful tool in structure elucidation of organic compounds.[3] This is possible with CI, because formation of [M+1]+ eliminates a stable molecule, which can be used to guess the functional groups present.[3] Besides that, CI facilitates the ability to detect the molecular ion peak, due to less extensive fragmentation.[3] Chemical ionization can also be used to identify and quantify an analyte present in a sample, by coupling chromatographic separation techniques to CI[3] such as gas chromatography (GC), high performance liquid chromatography (HPLC) and capillary electrophoresis (CE). This allows selective ionization of an analyte from a mixture of compounds, where accurate and precised results can be obtained.
Variants
Negative chemical ionization
Chemical ionization for gas phase analysis is either positive or negative.[9] Almost all neutral analytes can form positive ions through the reactions described above.
In order to see a response by negative chemical ionization (NCI, also NICI), the analyte must be capable of producing a negative ion (stabilize a negative charge) for example by electron capture ionization. Because not all analytes can do this, using NCI provides a certain degree of selectivity that is not available with other, more universal ionization techniques (EI, PCI). NCI can be used for the analysis of compounds containing acidic groups or electronegative elements (especially halogens).[5]:23Moreover, negative chemical ionization is more selective and demonstrates a higher sensitivity toward oxidizing agents and alkylating agents.[10]
Because of the high electronegativity of halogen atoms, NCI is a common choice for their analysis. This includes many groups of compounds, such as PCBs,[10] pesticides, and fire retardants.[10] Most of these compounds are environmental contaminants, thus much of the NCI analysis that takes place is done under the auspices of environmental analysis. In cases where very low limits of detection are needed, environmental toxic substances such as halogenated species, oxidizing and alkylating agents[9] are frequently analyzed using an electron capture detector coupled to a gas chromatograph.
Negative ions are formed by resonance capture of a near-thermal energy electron, dissociative capture of a low energy electron and via ion-molecular interactions such as proton transfer, charge transfer and hydride transfer.[9] Compared to the other methods involving negative ion techniques, NCI is quite advantageous, as the reactivity of anions can be monitored in the absence of a solvent. Electron affinities and energies of low-lying valencies can be determined by this technique as well.[9]
Charge-exchange chemical ionization
This is also similar to CI and the difference lies in the production of a radical cation with an odd number of electrons. The reagent gas molecules are bombarded with high energy electrons and the product reagent gas ions abstract electrons from the analyte to form radical cations. The common reagent gases used for this technique are toluene, benzene, NO, Xe, Ar and He.
Careful control over the selection of reagent gases and the consideration toward the difference between the resonance energy of the reagent gas radical cation and the ionization energy of the analyte can be used to control fragmentation.[6] The reactions for charge-exchange chemical ionization are as follows.
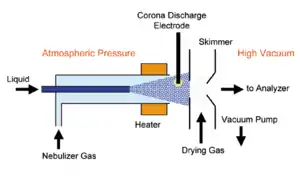
Atmospheric-pressure chemical ionization
Chemical ionization in an atmospheric pressure electric discharge is called atmospheric pressure chemical ionization (APCI), which usually uses water as the reagent gas. An APCI source is composed of a liquid chromatography outlet, nebulizing the eluent, a heated vaporizer tube, a corona discharge needle and a pinhole entrance to 10−3 torr vacuum.[8] The analyte is a gas or liquid spray and ionization is accomplished using an atmospheric pressure corona discharge. This ionization method is often coupled with high performance liquid chromatography where the mobile phase containing eluting analyte sprayed with high flow rates of nitrogen or helium and the aerosol spray is subjected to a corona discharge to create ions. It is applicable to relatively less polar and thermally less stable compounds. The difference between APCI and CI is that APCI functions under atmospheric pressure, where the frequency of collisions is higher. This enables the improvement in sensitivity and ionization efficiency.[6]
References
- Fales HM, Milne GW, Pisano JJ, Brewer HB, Blum MS, MacConnell JG, Brand J, Law N (1972). "Biological applications of electron ionization and chemical ionization mass spectrometry". Recent Prog. Horm. Res. 28: 591–626. PMID 4569234.
- Field, Frank H. (2002). "Chemical ionization mass spectrometry". Accounts of Chemical Research. 1 (2): 42–49. doi:10.1021/ar50002a002.
- Alex. G. Harrison (15 June 1992). Chemical Ionization Mass Spectrometry, Second Edition. CRC Press. pp. 1–. ISBN 978-0-8493-4254-7.
- Hunt, Donald F.; McEwen, Charles N.; Harvey, T. Michael. (2002). "Positive and negative chemical ionization mass spectrometry using a Townsend discharge ion source". Analytical Chemistry. 47 (11): 1730–1734. doi:10.1021/ac60361a011.
- de Hoffmann, Edmond; Vincent Stroobant (2003). Mass Spectrometry: Principles and Applications (Second ed.). Toronto: John Wiley & Sons, Ltd. p. 14. ISBN 978-0-471-48566-7.
- Dass, Chhabil (2007). Fundamentals of contemporary mass spectrometry ([Online-Ausg.]. ed.). Hoboken, N.J.: Wiley-Interscience. ISBN 9780470118498.
- Sahba Ghaderi; P. S. Kulkarni; Edward B. Ledford; Charles L. Wilkins; Michael L. Gross (1981). "Chemical ionization in Fourier transform mass spectrometry". Analytical Chemistry. 53 (3): 428–437. doi:10.1021/ac00226a011.
- Byrdwell, William Craig (2001-04-01). "Atmospheric pressure chemical ionization mass spectrometry for analysis of lipids". Lipids. 36 (4): 327–346. doi:10.1007/s11745-001-0725-5. ISSN 0024-4201. PMID 11383683.
- Dougherty R.C. (1981). "Negative chemical ionization mass spectrometry: applications in environmental analytical chemistry". Biomed. Mass Spectrom. 8 (7): 283–292. doi:10.1002/bms.1200080702. PMID 7025931.
- Dougherty, Ralph C. (2002). "Negative chemical ionization mass spectrometry". Analytical Chemistry. 53 (4): 625–636. doi:10.1021/ac00227a003.
Bibliography
- Harrison, Alex. G. (1992). Chemical ionization mass spectrometry (2. ed.). Boca Raton, Fla. [u.a.]: CRC Press. ISBN 9780849342547.
- Hunt, Donald F.; McEwen, Charles N.; Harvey, T. Michael. (2002). "Positive and negative chemical ionization mass spectrometry using a Townsend discharge ion source". Analytical Chemistry. 47 (11): 1730–1734. doi:10.1021/ac60361a011.
- Dass, Chhabil (2007). Fundamentals of contemporary mass spectrometry ([Online-Ausg.]. ed.). Hoboken, N.J.: Wiley-Interscience. ISBN 9780470118498.