Chloramines
Chloramines refer to derivatives of ammonia and organic amines wherein one or more N-H bonds have been replaced by N-Cl bonds. Two classes of compounds are considered: inorganic chloramines and organic chloramines.
Inorganic chloramines
Inorganic chloramines comprise three compounds: monochloramine (NH2Cl), dichloramine (NHCl2), and nitrogen trichloride (NCl3). Monochloramine is of broad significance as a disinfectant for water.[1]
Organic chloramines
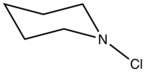
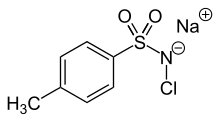
A variety of organic chloramines are useful in organic synthesis. Examples include N-chloromorpholine (ClN(CH2CH2)2O), N-chloropiperidine, and N-chloroquinuclidinium chloride.[4]
Chloramines are commonly produced by the action of bleach on secondary amines:
- R2NH + NaOCl → R2NCl + NaOH
Tert-butyl hypochlorite is often used instead of bleach:[5]
- R2NH + t-BuOCl → R2NCl + t-BuOH
Swimming pools
Chloramines also refers to any chloramine formed by chlorine reacting with ammonia introduced into swimming pools by human perspiration, saliva, mucus, urine, and other biologic substances, and by insects and other pests.[6] Chloramines are responsible for the "chlorine smell" of pools, as well as skin and eye irritation. These problems are the result of insufficient levels of free available chlorine, and indicate a pool that must be "shocked" by the addition of 5-10 times the normal amount of chlorine.[7]
References
- Lawrence, Stephen A. (2004). Amines: Synthesis, Properties and Applications. Cambridge University Press. p. 172. ISBN 9780521782845.
- Claxton, George P.; Allen, Lloyd; Grisar, J. Martin (1977). "2,3,4,5-Tetrahydropyridine Trimer". Organic Syntheses. 56: 118. doi:10.15227/orgsyn.056.0118.
- Campbell, Malcolm M.; Johnson, Graham. (1978). "Chloramine T and Related N-halogeno-N-metallo Reagents". Chemical Reviews. 78: 65–79. doi:10.1021/cr60311a005.
- Lindsay Smith, J. R.; McKeer, L. C.; Taylor, J. M. "4-Chlorination of Electron-Rich Benzenoid Compounds: 2,4-Dichloromethoxybenzene". Organic Syntheses. 67: 222. doi:10.15227/orgsyn.067.0222.; Collective Volume, 8, p. 167
- Herranz, Eugenio; Sharpless, K. Barry (1983). "Osmium-catalyzed Vicinal Oxyamination of Olefins by N-chloro-N-Argentocarbamates: Ethyl Threo-[1-(2-hydroxy-1,2-diphenylethyl)]carbamate". Org. Synth. 61: 93. doi:10.15227/orgsyn.061.0093.
- "Controlling Chloramines in Indoor Swimming Pools". NSW Government Health. 3 December 2012. Retrieved 20 February 2013.
- David Short, Fran J. Donegan (2012). Pools and Spas: Planning, Designing, Maintaining, Landscaping. Upper Saddle River, NJ: Creative Homeowner. p. 239. ISBN 978-1-58011-391-5.
