Chlorate
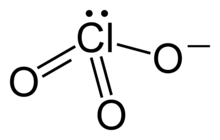
The chlorate anion has the formula ClO−
3. In this case, the chlorine atom is in the +5 oxidation state. "Chlorate" can also refer to chemical compounds containing this anion; chlorates are the salts of chloric acid. "Chlorate", when followed by a Roman numeral in parentheses, e.g. chlorate (VII), refers to a particular oxyanion of chlorine.
 | |
 | |
| Names | |
|---|---|
| Other names
Chlorate(V) | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI |
|
| ChemSpider | |
| 1491 | |
PubChem CID |
|
| UNII | |
| UN number | 1461 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| ClO3− | |
| Molar mass | 83.4512 |
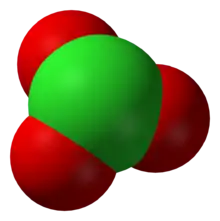
| Structure | |
| Trigonal pyramidal | |
| Hazards | |
| Main hazards | oxidation agent |
| Related compounds | |
Other anions |
|
Related compounds |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
As predicted by valence shell electron pair repulsion theory, chlorate anions have trigonal pyramidal structures.
Chlorates are powerful oxidizers and should be kept away from organics or easily oxidized materials. Mixtures of chlorate salts with virtually any combustible material (sugar, sawdust, charcoal, organic solvents, metals, etc.) will readily deflagrate. Chlorates were once widely used in pyrotechnics for this reason, though their use has fallen due to their instability. Most pyrotechnic applications that formerly used chlorates now use the more stable perchlorates instead.
Structure and bonding
The chlorate ion cannot be satisfactorily represented by just one Lewis structure, since all the Cl–O bonds are the same length (1.49 Å in potassium chlorate[1]), and the chlorine atom is hypervalent. Instead, it is often thought of as a hybrid of multiple resonance structures:

Preparation
Laboratory
Metal chlorates can be prepared by adding chlorine to hot metal hydroxides like KOH:
- 3 Cl2 + 6 KOH → 5 KCl + KClO3 + 3 H2O
In this reaction, chlorine undergoes disproportionation, both reduction and oxidation. Chlorine, oxidation number 0, forms chloride Cl− (oxidation number −1) and chlorate(V) ClO−
3 (oxidation number +5). The reaction of cold aqueous metal hydroxides with chlorine produces the chloride and hypochlorite (oxidation number +1) instead.
Industrial
The industrial scale synthesis for sodium chlorate starts from aqueous sodium chloride solution (brine) rather than chlorine gas. If the equipment for electrolysis allows for the mixing of the chlorine and the sodium hydroxide, then the disproportionation reaction described above occurs. The heating of the reactants to 50–70 °C is performed by the electrical power used for electrolysis.
Natural occurrence
A recent study has discovered the presence of natural chlorate deposits around the world, with relatively high concentrations found in arid and hyper-arid regions.[2] The chlorate was also measured in rainfall samples with the amount of chlorate similar to perchlorate. It is suspected that chlorate and perchlorate may share a common natural formation mechanism and could be a part of the chlorine biogeochemistry cycle. From a microbial standpoint, the presence of natural chlorate could also explain why there is a variety of microorganisms capable of reducing chlorate to chloride. Further, the evolution of chlorate reduction may be an ancient phenomenon as all perchlorate reducing bacteria described to date also utilize chlorate as a terminal electron acceptor.[3] It should be clearly stated, that currently no chlorate-dominant minerals are known. This means that the chlorate anion exists only as a substitution in the known mineral species, or – eventually – is present in the pore-filling solutions.[4]
In 2011, a study of the Georgia Institute of technology unveiled the presence of magnesium chlorate on the planet Mars.[5]
Compounds (salts)
Examples of chlorates include
- potassium chlorate, KClO3
- sodium chlorate, NaClO3
- magnesium chlorate, Mg(ClO3)2
Other oxyanions
If a Roman numeral in brackets follows the word "chlorate", this indicates the oxyanion contains chlorine in the indicated oxidation state, namely:
| Common name | Stock name | Oxidation state | Formula |
|---|---|---|---|
| Hypochlorite | Chlorate(I) | +1 | ClO− |
| Chlorite | Chlorate(III) | +3 | ClO− 2 |
| Chlorate | Chlorate(V) | +5 | ClO− 3 |
| Perchlorate | Chlorate(VII) | +7 | ClO− 4 |
Using this convention, "chlorate" means any chlorine oxyanion. Commonly, "chlorate" refers only to chlorine in the +5 oxidation state.
Toxicity
Chlorates are relatively toxic, though they form generally harmless chlorides on reduction.
References
- J. Danielsen; A. Hazell; F. K. Larsen (1981). "The structure of potassium chlorate at 77 and 298 K". Acta Crystallogr. B. 37 (4): 913–915. doi:10.1107/S0567740881004573.
- Rao, B.; Hatzinger, P. B.; Böhlke, J. K.; Sturchio, N. C.; Andraski, B. J.; Eckardt, F. D.; Jackson, W. (2010). "Natural Chlorate in the Environment: Application of a New IC-ESI/MS/MS Method with a Cl18O3− Internal Standard". Environ. Sci. Technol. 44 (22): 8429–8434. Bibcode:2010EnST...44.8429R. doi:10.1021/es1024228. PMID 20968289.
- Coates, J. D.; Achenbach, L. A. (2004). "Microbial perchlorate reduction: rocket-fuelled metabolism". Nature Reviews Microbiology. 2 (July): 569–580. doi:10.1038/nrmicro926. PMID 15197392. S2CID 21600794.
- http://www.mindat.org
- https://www.letemps.ch/sciences/2015/09/28/eau-liquide-reperee-pentes-martiennes
External links
| Wikimedia Commons has media related to Chlorate ion. |
- . Encyclopædia Britannica. 6 (11th ed.). 1911. p. 254.