Chlorine peroxide
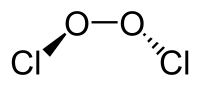
Chlorine peroxide (also known as dichlorine dioxide or ClO dimer) is a molecular compound with formula ClOOCl.[1] Chemically, it is a dimer of the chlorine monoxide radical (ClO·). It is important in the formation of the ozone hole.[2] Chlorine peroxide catalytically converts ozone into oxygen when it is irradiated by ultraviolet light.[3]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Chlorine peroxide | |
| Other names
Chlorine(I) oxide; ClO dimer | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| Cl2O2 | |
| Molar mass | 102.905 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Production
Chlorine peroxide can be produced by laser or ultraviolet photolysis of the chlorine molecule with ozone.[1] The lasers used to break up the chlorine molecule into atoms can be an excimer laser at 248, 308, or 352 nm wavelength.[3] Difluorodichloromethane (CF2Cl2) can also act as a source of chlorine atoms for the formation of the peroxide.[1] Microwave discharge can also break up chlorine molecules into atoms that react with ozone to make chlorine peroxide.[3]
- Cl2 + hν → 2Cl
- Cl + O3 → O2 + ClO·
- 2ClO· + M → ClOOCl + M
- ClOOCl + hν → Cl + ClO2
- ClO2 + M → Cl + O2
Properties
Chlorine peroxide absorbs ultraviolet light with a maximum absorbing wavelength of 245 nm. It also absorbs longer wavelengths up to 350 nm to a lesser extent. This is important as ozone absorbs up to 300 nm.[1]
The Cl−O bond length is 1.704 Å, and the O−O bond is 1.426 Å long.[4] The ClOO bond angle is 110.1°, and the dihedral angle between the two Cl−O−O planes is 81°[4]
References
- Pope, Francis D.; Jaron C. Hansen; Kyle D. Bayes; Randall R. Friedl; Stanley P. Sander (2007). "Ultraviolet Absorption Spectrum of Chlorine Peroxide, ClOOCl". The Journal of Physical Chemistry A. 111 (20): 4322–4332. Bibcode:2007JPCA..111.4322P. doi:10.1021/jp067660w. ISSN 1089-5639. PMID 17474723.
- Lin, Jim J.; Andrew F Chen; Yuan T. Lee (2011). "UV Photolysis of ClOOCl and the Ozone Hole". Chemistry: An Asian Journal. 6 (7): 1664–1678. doi:10.1002/asia.201100151. ISSN 1861-4728. PMID 21538907.
- Chen HY, Lien CY, Lin WY, Lee YT, Lin JJ (2009). "UV Absorption Cross Sections of ClOOCl Are Consistent with Ozone Degradation Models". Science. 324 (5928): 781–784. Bibcode:2009Sci...324..781C. doi:10.1126/science.1171305. ISSN 0036-8075. PMID 19423825.
- Inglese, S.; G. Granucci; T. Laino; M. Persico (2005). "Photodissociation Dynamics of Chlorine Peroxide Adsorbed on Ice". The Journal of Physical Chemistry B. 109 (16): 7941–7947. doi:10.1021/jp044368k. ISSN 1520-6106. PMID 16851927.
