Chlorine perchlorate
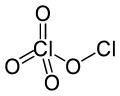
Chlorine perchlorate is a chemical compound with the formula Cl2O4. This chlorine oxide is an asymmetric oxide, with one chlorine atom in +1 oxidation state and the other +7, with proper formula ClOClO3. It is produced by the photolysis of chlorine dioxide (ClO2) at room temperature by 436 nm ultraviolet light :[2][3][4]
- 2 ClO2 → ClOClO3
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Chloro perchlorate[1] | |||
| Systematic IUPAC name
Chloro perchlorate[1] | |||
| Other names
Chlorine (I,VII) oxide Dichlorine tetroxide | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
PubChem CID |
|||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| Cl2O4 | |||
| Molar mass | 134.90 g·mol−1 | ||
| Appearance | Pale green liquid | ||
| Density | 1.81 g cm−3 | ||
| Melting point | −117 °C (−179 °F; 156 K) | ||
| Boiling point | 20 °C (68 °F; 293 K) (decomposes) | ||
| Reacts | |||
| Hazards | |||
| Main hazards | oxidizer | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Chlorine perchlorate can also be made by the following reaction at −45 °C.
- CsClO4 + ClOSO2F → Cs(SO3)F + ClOClO3
Properties
Chlorine perchlorate is a pale greenish liquid. It is less stable than ClO2 (chlorine dioxide) and decomposes at room temperature to give O2 (oxygen), Cl2 (chlorine) and Cl2O6 (dichlorine hexoxide):
- 2 ClOClO3 → O2 + Cl2 + Cl2O6
Chlorine perchlorate reacts with metal chlorides to form chlorine and the corresponding anhydrous perchlorate:
- CrO2Cl2 + 2 ClOClO3 → 2 Cl2 + CrO2(ClO4)2
- TiCl4 + 4 ClOClO3 → 4 Cl2 + Ti(ClO4)4
- 2 AgCl + 2 ClOClO3 → 2 AgClO4 + Cl2
Reactions
| Reactant | Conditions | Products |
|---|---|---|
| — | Heat | dichlorine hexoxide (80%), chlorine dioxide, chlorine, oxygen |
| — | Ultraviolet Light | dichlorine heptoxide, chlorine, oxygen[4] |
| caesium iodide | -45 °C | Cs[I(OClO3)4][note 1] |
| ClOSO2F or ClF | — | MClO4(M = Cs or NO2)[note 2] |
| bromine | -45 °C | bromine perchlorate (BrOClO3)[note 2] |
| iodine(0.33mol) | -50 °C | I(OClO3)3[note 3][5] |
Notes:
- 1. Cs[I(OClO3)4] is a pale yellow salt which is stable at room temperature. It has a square IO4 unit.
- 2. MClO4(M = Cs or NO2) reacts with BrOSO2F at -20 °C and produces bromine perchlorate (BrOClO3). Bromine perchlorate then reacts with hydrogen bromide (HBr) at -70 °C and produces elemental bromine (Br2) and perchloric acid (HClO4).
- 3. So far, all attempts to form iodine perchlorate(IOClO3) have failed, because the iodine atom oxidizes to +3 oxidation state immediately.
References
- "Chloro Perchlorate - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information.
- A. J. Schell-Sorokin; D. S. Bethune; J. R. Lankard; M. M. T. Loy; P. P. Sorokin (1982). "Chlorine perchlorate a major photolysis product of chlorine dioxide". J. Phys. Chem. 86 (24): 4653–4655. doi:10.1021/j100221a001.
- M. I. Lopez; J. E. Sicre (1988). "Ultraviolet spectrum of chlorine perchlorate". J. Phys. Chem. 92 (2): 563–564. doi:10.1021/j100313a062.
- Rao, Balaji; Anderson, Todd A.; Redder, Aaron; Jackson, W. Andrew (2010-04-15). "Perchlorate Formation by Ozone Oxidation of Aqueous Chlorine/Oxy-Chlorine Species: Role of ClxOy Radicals". Environmental Science & Technology. 44 (8): 2961–2967. doi:10.1021/es903065f. ISSN 0013-936X. PMID 20345093.
- Gomberg, M. (1923-02-01). "The Reaction Between Silver Perchlorate and Iodine. Chlorine Tetra-Oxide". Journal of the American Chemical Society. 45 (2): 398–421. doi:10.1021/ja01655a017. ISSN 0002-7863.
| HClO4 | He | ||||||||||||||||
| LiClO4 | Be(ClO4)2 | B(ClO 4)− 4 B(ClO4)3 |
ROClO3 | N(ClO4)3 NH4ClO4 NOClO4 |
O | FClO4 | Ne | ||||||||||
| NaClO4 | Mg(ClO4)2 | Al(ClO4)3 | Si | P | S | ClO− 4 ClOClO3 Cl2O7 |
Ar | ||||||||||
| KClO4 | Ca(ClO4)2 | Sc(ClO4)3 | Ti(ClO4)4 | VO(ClO4)3 VO2(ClO4) |
Cr(ClO4)3 | Mn(ClO4)2 | Fe(ClO4)3 | Co(ClO4)2, Co(ClO4)3 |
Ni(ClO4)2 | Cu(ClO4)2 | Zn(ClO4)2 | Ga(ClO4)3 | Ge | As | Se | Br | Kr |
| RbClO4 | Sr(ClO4)2 | Y(ClO4)3 | Zr(ClO4)4 | Nb(ClO4)5 | Mo | Tc | Ru | Rh(ClO4)3 | Pd(ClO4)2 | AgClO4 | Cd(ClO4)2 | In(ClO4)3 | Sn(ClO4)4 | Sb | TeO(ClO4)2 | I | Xe |
| CsClO4 | Ba(ClO4)2 | Hf(ClO4)4 | Ta(ClO4)5 | W | Re | Os | Ir | Pt | Au | Hg2(ClO4)2, Hg(ClO4)2 |
Tl(ClO4), Tl(ClO4)3 |
Pb(ClO4)2 | Bi(ClO4)3 | Po | At | Rn | |
| FrClO4 | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La | Ce(ClO4)x | Pr | Nd | Pm | Sm(ClO4)3 | Eu(ClO4)3 | Gd(ClO4)3 | Tb(ClO4)3 | Dy(ClO4)3 | Ho(ClO4)3 | Er(ClO4)3 | Tm(ClO4)3 | Yb(ClO4)3 | Lu(ClO4)3 | |||
| Ac | Th(ClO4)4 | Pa | UO2(ClO4)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.