Chlorotoluene
Chlorotoluene is a group of three isomeric chemical compounds. They (ortho-chlorotoluene, meta-chlorotoluene, and para-chlorotoluene) consist of a disubstituted benzene ring with one chlorine atom and one methyl group.
Properties
The isomers differ in the location of the chlorine, but have the same chemical formula. All have very similar boiling points, although p-chlorotoluene has a much higher melting point due to a more tightly-packed crystal structure.
| Chlorotoluene isomers | ||||
|---|---|---|---|---|
| General | ||||
| Common name | o-chlorotoluene | m-chlorotoluene | p-chlorotoluene | |
| Structure | 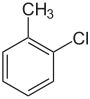 |
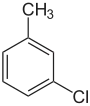 |
 | |
| Systematic name | 1-chloro-2-methylbenzene | 1-chloro-3-methylbenzene | 1-chloro-4-methylbenzene | |
| Molecular formula | C7H7Cl (C6H4ClCH3) | |||
| Molar mass | 126.586 g/mol | |||
| Appearance | colorless liquid | |||
| CAS number | [95-49-8] | [108-41-8] | [106-43-4] | |
| Properties | ||||
| Density and phase | 1.073 g/ml, liquid | 1.072 g/ml, liquid | 1.069 g/ml, liquid | |
| Solubility in water | practically insoluble | |||
| Other solubilities | Soluble in non-polar solvents such as aromatic hydrocarbons | |||
| Melting point | −35 °C (−31 °F; 238 K) | −47 °C (−52.6 °F; 226 K) | 7 °C (44.6 °F; 280 K) | |
| Boiling point | 159 °C (318.2 °F; 432 K) | 162 °C (323.6 °F; 435 K) | 162 °C (323.6 °F; 435 K) | |
| Magnetic susceptibility | -81.98 x 10−6 cm3/mol | -80.07 x 10−6 cm3/mol | -80.07 x 10−6 cm3/mol | |
Benzyl chloride is an isomer, which has a chlorine substituted for one of the hydrogens of toluene's methyl group, and it is sometimes named α-chlorotoluene.
Uses
2- and 4-Chlorotoluene are precursors to the corresponding benzyl chloride (ClC6H4CH2Cl), benzaldehyde (ClC6H4CHO), and benzoyl chloride (ClC6H4C(O)Cl).[1]
References
- Beck, Uwe; Löser, Eckhard (2011). "Chlorinated Benzenes and Other Nucleus-Chlorinated Aromatic Hydrocarbons". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.o06_o03.
