Cinacalcet
Cinacalcet, sold under the brand name Sensipar among others, is a medication used to treat secondary hyperparathyroidism, parathyroid carcinoma, and primary hyperparathyroidism.[4][5][6]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Sensipar, Mimpara |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a605004 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 20 to 25% increases if taken with food |
| Protein binding | 93 to 97% |
| Metabolism | Hepatic (CYP3A4-, CYP2D6- and CYP1A2-mediated) |
| Elimination half-life | 30 to 40 hours |
| Excretion | Renal (80%) and fecal (15%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| PubChem SID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.208.116 |
| Chemical and physical data | |
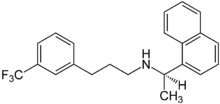
| Formula | C22H22F3N |
| Molar mass | 357.420 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
The most common side effects include nausea (feeling sick) and vomiting.[6]
Cinacalcet acts as a calcimimetic (i.e., it mimics the action of calcium on tissues) by allosteric activation of the calcium-sensing receptor that is expressed in various human organ tissues.
Cinacalcet was approved in the United States in March 2004,[4][7][8] and in the European Union in October 2004.[6][3] It was the first allosteric G protein-coupled receptor modulator to enter the pharmaceutical market.[9] In 2013, cinacalcet was the 76th top prescribed medicine in the United States.[10][11]
Medical uses
In the United States, cinacalcet is indicated for the treatment of secondary hyperparathyroidism in people with chronic kidney disease on dialysis and hypercalcemia in people with parathyroid carcinoma.[4][12] Cinacalcet can also be used to treat severe hypercalcemia in patients with primary hyperparathyroidism who are unable to undergo parathyroidectomy.[4][13]
In the European Union cinacalcet is indicated for:
- the treatment of secondary hyperparathyroidism (HPT) in adults with end stage renal disease (ESRD) on maintenance dialysis therapy.[6]
- the treatment of secondary hyperparathyroidism (HPT) in children aged three years and older with end stage renal disease (ESRD) on maintenance dialysis therapy in whom secondary HPT is not adequately controlled with standard of care therapy.[6]
- part of a therapeutic regimen including phosphate binders and/or vitamin D sterols, as appropriate.[6]
- the treatment of parathyroid carcinoma and primary hyperparathyroidism in adults.[6]
- the reduction of hypercalcaemia in adults with:
Pregnancy and lactation
Cinacalcet has pregnancy category C in the US, meaning that adequate and well-controlled studies involving cinacalcet in pregnant women have not been done.[4][1]
Studies have not been done in lactating women; therefore it is not known whether cinacalcet is excreted into human milk.[4][1]
Contraindications
Hypocalcemia (decreased calcium levels) is a contraindication of cinacalcet. Those who have serum calcium levels less than 7.5 mg/dL should not be started on cinacalcet. Hypocalcemia symptoms include parathesias, myalgias, muscle cramping, tetany, and convulsions. Cinacalcet should not be administered until serum calcium levels are above 8.0 mg/dL and/or hypocalcemia symptoms are resolved.[4] Cinacalcet is not approved for pediatric use in the United States.[5][4][6]
Adverse effects
Common side effects of cinacalcet include stomach upset, vomiting, diarrhea, dizziness, nausea, weakness, and chest pain.[13]
Clinical trials conducted in the United States by Amgen to determine whether the drug is safe in children were halted by the U.S. Food and Drug Administration (FDA) in February 2013, following the death of a 14-year-old participant.[5][14]
Overdose
Serious side effects, including overdose symptoms, of cinacalcet include:[13]
- burning
- tingling
- unusual feelings of the lips, tongue, fingers, or feet
- muscle aches or cramps
- sudden tightening of muscles in hands, feet, face, or throat
- seizures
Interactions
Cinacalcet is a strong inhibitor of the liver enzyme CYP2D6 and is partially metabolized by CYP3A4 and CYP1A2. Dose adjustments may be necessary if people are treated with CYP3A4 and CYP1A2 inhibitors and medications that are metabolized by CYP2D6.[4][3]
Pharmacology
Mechanism of action
Cinacalcet is a drug that acts as a calcimimetic[4][6] (i.e. it mimics the action of calcium on tissues) by allosteric activation of the calcium-sensing receptor that is expressed in various human organ tissues. The calcium-sensing receptors on the surface of the chief cell of the parathyroid gland is the principal negative regulator of parathyroid hormone secretion.[15] Cinacalcet increases the sensitivity of calcium receptors on parathyroid cells to reduce parathyroid hormone (PTH) levels and thus decrease serum calcium levels.[12]
References
- "Cinacalcet (Sensipar) Use During Pregnancy". Drugs.com. 19 July 2019. Retrieved 24 December 2019.
- "Sensipar Tablets". NPS MedicineWise. 1 May 2018. Archived from the original on 7 December 2019. Retrieved 7 December 2019.
- "Mimpara 30 mg Film-coated Tablets - Summary of Product Characteristics (SmPC)". electronic medicines compendium (emc). 8 July 2019. Archived from the original on 7 December 2019. Retrieved 7 December 2019.
- "Sensipar- cinacalcet hydrochloride tablet, coated". DailyMed. 5 December 2019. Retrieved 24 December 2019.
- "FDA Drug Safety Communication: Pediatric clinical studies of Sensipar (cinacalcet hydrochloride) suspended after report of death". U.S. Food and Drug Administration (FDA). 15 January 2016. Archived from the original on 24 December 2019. Retrieved 23 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "Mimpara EPAR". European Medicines Agency (EMA). 22 August 2019. Retrieved 23 December 2019. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- "Drug Approval Package: Sensipar (Cinacalcet HCI) NDA #021688". U.S. Food and Drug Administration (FDA). 7 December 2019. Archived from the original on 7 December 2019. Retrieved 7 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "Sensipar". U.S. Food and Drug Administration (FDA). Archived from the original on 7 December 2019. Retrieved 7 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - Bräuner-Osborne H, Wellendorph P, Jensen AA (2007). "Structure, pharmacology and therapeutic prospects of family C G-protein coupled receptors". Current Drug Targets. 8 (1): 169–84. doi:10.2174/138945007779315614. PMID 17266540.
- "U.S. Pharmaceutical Statistics". Drugs.com. February 2014. Retrieved 13 July 2020.
- "Sales Statistics for Sensipar Prescriptions". Drugs.com. 2 September 2020. Retrieved 8 September 2020.
- 2014 Nurses Drug Handbook (13th ed.). Burlington, MA: Jones & Bartlett Learning. 2014. pp. 245–6. ISBN 978-1-284-03115-7.
- "Cinacalcet". U.S. National Library of Medicine. Retrieved 29 October 2014.
- Edney A (February 26, 2013). "Amgen Pediatric Trials of Sensipar Halted by FDA After Death". Bloomberg Businessweek. Archived from the original on March 2, 2013. Retrieved February 26, 2013.
- "Cinacalcet".
External links
- "Cinacalcet". Drug Information Portal. U.S. National Library of Medicine.
- "Cinacalcet hydrochloride". Drug Information Portal. U.S. National Library of Medicine.