Crotalus cerastes
The sidewinder (Crotalus cerastes), also known as the horned rattlesnake and sidewinder rattlesnake,[3] is a venomous pit viper species belonging to the genus Crotalus (rattlesnakes) and is found in the desert regions of the southwestern United States and northwestern Mexico. The sidewinder reaches speeds of up to 18 mph (29 km/h). Three subspecies are currently recognized.[4]
| Sidewinder (Crotalus cerastes) | |
|---|---|
 | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Reptilia |
| Order: | Squamata |
| Suborder: | Serpentes |
| Family: | Viperidae |
| Genus: | Crotalus |
| Species: | C. cerastes |
| Binomial name | |
| Crotalus cerastes Hallowell, 1854 | |
 | |
| Synonyms | |
Description
A small species, adult specimens measure between 43 and 76 cm (17 and 30 in) in length.[3] Most adults are 50–80 cm (19.5–31.5 in) in length.[5] The females are larger than the males, which is unusual for this group of snakes.[6]
Usually, 21 rows of keeled dorsal scales occur midbody.[3][7] Males have 141 or fewer ventral scales; females have 144 or fewer.[3] It is sometimes referred to as the horned rattlesnake because of the raised supraocular scales above its eyes. This adaptation may help shade the eyes or prevent sand drifting over them as the snake lies almost buried in it.[6]
The color pattern consists of a ground color that may be cream, buff, yellowish-brown, pink, or ash gray, overlaid with 28-47 dorsal blotches subrhombic or subelliptical in shape.[5] In the nominate subspecies, the belly is white and the proximal lobe of the rattle is brown in adults. Klauber and Neill describe the ability of this species to display different coloration depending on the temperature—a process known as metachrosis.[3]
Common names
Sidewinder, horned rattlesnake, sidewinder rattlesnake, Mojave Desert sidewinder (for C. c. cerastes),[3] sidewinder rattler.[8]
Geographic range
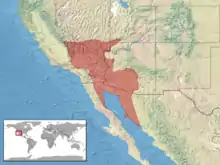
In the southwestern United States, this species is found in the desert region of south eastern California, southern Nevada, southwestern Utah, and western Arizona. In northwestern Mexico, it is found in western Sonora and eastern Baja California.
Conservation status
This species is classified as Least Concern on the IUCN Red List (v3.1, 2001).[1] Species are listed as such due to their wide distribution, presumed large population, or because they are unlikely to be declining fast enough to qualify for listing in a more threatened category. The population trend was stable when assessed in 2007.[9]
Behavior

The common name sidewinder alludes to its unusual form of locomotion, which is thought to give it traction on windblown desert sand, but this peculiar locomotor specialization is used on any substrate over which the sidewinder can move rapidly. As its body progresses over loose sand, it forms a letter J-shaped impression, with the tip of the hook pointing in the direction of travel. Sidewinding is also the primary mode of locomotion in other desert sand dwellers, such as the horned adder (Bitis caudalis) and Peringuey's adder (Bitis peringueyi), but many other snakes can assume this form of locomotion when on slick substrates (e.g., mud flats). Sidewinder rattlesnakes can use sidewinding to ascend sandy slopes by increasing the portion of the body in contact with the sand to match the reduced yielding force of the inclined sand, allowing them to ascend up to the maximum possible sand slope without slip.[10] In cybernetics, incorporating this control scheme into a snakebot can enable the robot to replicate sidewinding movement.[10]
The species is nocturnal during hot months and diurnal during the cooler months of its activity period, which is roughly from November to March (probably longer in the southern part of its range).
Juveniles use their tails to attract lizard prey, a behavior termed "caudal luring". Adults lose this behavior as they make the transition from lizard prey to their primary diet of desert rodents. Sidewinder juveniles appear to mimic both life stages of lepidopterans in their luring motions. Their fast luring motions resemble the fluttering of a moth, and their slower tail movements resemble a caterpillar. Both movements have been observed to attract prey lizards.[11]
Neonatal sidewinders engage in a remarkable behavioral homeothermy that has not been observed in any other type of snake.[12] Following birth, the neonates mass together in their natal burrow. Most often, gravid females select an east-facing, small-diameter rodent burrow for giving birth. For the first week or so of their lives, neonatal sidewinders plug the entrance to this burrow during daylight hours, forming a dynamic multiple-individual mass that takes advantage of the hot exterior environment and the cool interior of the burrow to maintain an average aggregate temperature of 32 °C (the optimal temperature for shedding). The dynamic mass of neonates modifies the thermal environment at the burrow entrance such that the young can occupy a location that would ordinarily become lethally hot for an individual neonate (or even an adult).[12] Because of the constant movements of the neonates, the aggregate assumes stable temperature properties reminiscent of a homeothermic organism (i.e., maintains tight temperature tolerance ± 2 °C).
Reproduction
Females produce up to 18 young, with an average of about 10 per litter. Like most other viperids, the young are born enveloped in thin embryonic membranes, from which they emerge shortly after being expelled from the mother. The young stay with their mother in a burrow for seven to 10 days, shed for the first time, then leave their natal burrow. During this time, the mother is thought to guard and protect them from predators.
Sidewinders mature at two to three years of age, are capable of reproducing annually, and give birth to live young. Some females skip reproductive opportunities.[13] Some might even skip two years if the food supply is scarce. Sidewinders mate in April through May and sometimes in fall. When the male and female mate, the male snake crawls along the female's back, rubbing her with his chin to stimulate or arouse her. The male then will wrap his tail around her tail, and then will try to bring their cloacae together. The cloaca is the posterior body opening through which snakes both excrete waste and reproduce. If the female wants to mate, she will lift her tail and allow him to mate with her. The snakes can mate for several hours, and if one of the snakes decides to move, the other is dragged along. Females might mate with several males in a season. Females give birth to five to 18 young in late summer to early fall. The young are born six to eight inches long. The birth takes only two to three hours altogether. Within a few minutes of being born, the newborn sidewinder escapes from a thin, transparent membrane. The young stay at their natal burrow for seven to ten days until they shed,[12] and then they disappear and have no future contact with their mother or their litter mates. While the density of sidewinders can be up to one individual per hectare, they rarely encounter each other except during mating season.[13]
Sidewinders have an extraordinarily accelerated lifecycle, with natural life expectancies of females of about five years.[13] Males may live quite a bit longer (maximum known natural lifespan of 13 years). Sidewinders can live more than 20 years when well fed in captivity (even females). Thus, energetics apparently factor heavily into natural female mortality,[13] whereas predation might be the primary pressure on males. In the wild, females often die of exhaustion after giving birth, but the lives of sidewinders are also cut short by predation, diseases, and vehicle encounters.
Venom
These snakes are venomous, but possess a weaker venom than many other rattlesnakes. This, together with the smaller size of their venom glands, makes them less dangerous than their larger relatives. Still, any rattlesnake bite can be fatal and should be taken seriously and medical attention sought immediately.
Norris (2004) lists the following venom yields: 33 mg average and 63 mg maximum (Klauber, 1956), and 30 mg average and 80 mg maximum (Glenn & Straight, 1982).[14] Brown (1973) gives a venom yield of 33 mg (Klauber, 1956) and LD50 values for mice of 2.6 mg/kg IV, 3.0, 4.0, 2.3 mg/kg IP and 5.5 mg/kg SC for toxicity. With these figures, Brown calculated that the LD50 for an adult human being weighing 70 kg would be 385 mg (SC).[15]
Envenomation can cause pain, swelling, hemorrhagic bleb formation and ecchymosis (i.e., bruising). Swelling, while not particularly severe, occasionally may involve entire limbs and the trunk.
Envenomation's systemic symptoms include nausea, dizziness, chills, coagulopathy (blood disorders) and shock.[14] Klauber (1997) includes an account of a man who had been bitten on the first joint of the index finger of the right hand, with only a single fang penetrating. Although the bite was described as no more painful than a pin prick, a doctor was seen within about 25 minutes, and 10 cc of antivenin were administered. Within 2.5 hours, his entire arm was swollen and the pain was severe, "as if the arm were soaked in a bucket of boiling oil."[16]
Ovine-derived antivenom, CroFab, for North American pit viper envenomation has been widely available since 2001. Consultation with a local expert or regional poison control center should be obtained before administering antivenom. The previous antivenin (ACP) is no longer manufactured.
Subspecies
| Subspecies[4] | Taxon author[4] | Common name[16] | Geographic range[3] |
|---|---|---|---|
| C. c. cerastes | Hallowell, 1854 | Mojave Desert sidewinder | In the United States in the desert areas from northeastern Los Angeles County and San Bernardino County, California, northward to southern Mono County, California, east across Nevada to Washington County, Utah, and south through La Paz County, Arizona in desert lowlands at elevations between 152 and 1,829 m |
| C. c. cercobombus | Savage & Cliff, 1953 | Sonoran Desert sidewinder | In the United States from Yuma, Maricopa, Pima and Pinal Counties in Arizona, southward into Sonora, Mexico |
| C. c. laterorepens | Klauber, 1944 | Colorado Desert sidewinder | The desert areas in the United States from central and eastern Riverside County, California, to Pinal County, Arizona, south to northwestern Sonora in Mexico, and northwest to northeastern Baja California, from the Colorado River to the desert foothills at elevations between 152 and 610 m |
References
- Frost, D.R.; Hammerson, G.A. & Gadsden, H. (2007). "Crotalus cerastes". IUCN Red List of Threatened Species. 2007. Retrieved 5 June 2017.CS1 maint: ref=harv (link)
- McDiarmid RW, Campbell JA, Touré T. 1999. Snake Species of the World: A Taxonomic and Geographic Reference, vol. 1. Herpetologists' League. 511 pp. ISBN 1-893777-00-6 (series). ISBN 1-893777-01-4 (volume).
- Wright AH, Wright AA. 1957. Handbook of Snakes. Comstock Publishing Associates. (7th printing, 1985). 1105 pp. ISBN 0-8014-0463-0.
- "Crotalus cerastes". Integrated Taxonomic Information System. Retrieved 5 February 2007.
- Campbell JA, Lamar WW. 2004. The Venomous Reptiles of the Western Hemisphere. Comstock Publishing Associates, Ithaca and London. 870 pp. 1500 plates. ISBN 0-8014-4141-2.
- Stidworthy J. 1974. Snakes of the World. Grosset & Dunlap Inc. 160 pp. ISBN 0-448-11856-4.
- Behler JL, King FW. 1979. The Audubon Society Field Guide to North American Reptiles and Amphibians. New York: Alfred A. Knopf. 743 pp. LCCCN 79-2217. ISBN 0-394-50824-6.
- Carr A. 1963. The Reptiles. Life Nature Library. Time-Life Books, New York. LCCCN 63-12781.
- 2001 Categories & Criteria (version 3.1) at the IUCN Red List. Accessed 13 September 2007.
- Marvi, Hamidreza; Gong, Chaohui; Gravish, Nick; Astley, Henry; Travers, Matthew; Hatton, Ross L.; Mendelson, Joseph R. III; Choset, Howie; Hu, David L.; Goldman, Daniel I. (10 October 2014). "Sidewinding with Minimal Slip: Snake and Robot Ascent of Sandy Slopes". Science. 346 (6206): 224–9. arXiv:1410.2945. doi:10.1126/science.1255718. PMID 25301625. S2CID 23364137.
- Reiserer, R. S. and G. W. Schuett (2008) Aggressive mimicry in neonates of the sidewinder rattlesnake, Crotalus cerastes (Serpentes: Viperidae): stimulus control and visual perception of prey luring. Biological Journal of the Linnean Society 95:81-91(11).
- Reiserer, R. S., G. W. Schuett, and R. L. Early (2008) Dynamic aggregations of newborn sibling rattlesnakes exhibit stable thermoregulatory properties. Journal of Zoology 274:277-283(7).
- Reiserer, R. S. 2001. Evolution of Life Histories in Rattlesnakes, University of California, Berkeley. Advisors: Harry W. Greene and James L. Patton. ProQuest Digital Dissertations: http://wwwlib.umi.com/dissertations/gateway.
- Norris R. 2004. Venom Poisoning in North American Reptiles. In Campbell JA, Lamar WW. 2004. The Venomous Reptiles of the Western Hemisphere. Comstock Publishing Associates, Ithaca and London. 870 pp. 1500 plates. ISBN 0-8014-4141-2.
- Brown JH. 1973. Toxicology and Pharmacology of Venoms from Poisonous Snakes. Springfield, Illinois: Charles C. Thomas. 184 pp. LCCCN 73-229. ISBN 0-398-02808-7.
- Klauber LM. 1997. Rattlesnakes: Their Habitats, Life Histories, and Influence on Mankind. Second Edition. First published in 1956, 1972. University of California Press, Berkeley. ISBN 0-520-21056-5.
Further reading
- Hallowell, E. Descriptions of new Reptiles from California. Proc. Acad. Nat. Sci. Philadelphia 7: 91-97.
External links
| Wikimedia Commons has media related to Crotalus cerastes. |
- Crotalus cerastes at the Reptarium.cz Reptile Database. Accessed 3 August 2007.
- Crotalus cerastes cerastes at California Reptiles and Amphibians. Accessed 5 February 2007.
- Crotalus cerastes at WildHerps. Accessed 5 February 2007.
- The Sidewinder at VenomousReptiles.org. Accessed 5 February 2007.
- Mojave Desert Sidewinder at Bird and Hike . com. Accessed 5 February 2007.
