Curium hydroxide
Curium hydroxide [Cm(OH)3] is a radioactive compound first discovered in measurable quantities in 1947. It is composed of a single curium atom, and three hydroxide groups. It was the first curium compound ever isolated.[3][4]
 | |
| Names | |
|---|---|
| IUPAC name
Curium hydroxide | |
| Systematic IUPAC name
Curium(3+) oxidanide | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| CmH3O3 | |
| Molar mass | 298 g·mol−1 |
| Appearance | colorless or pale yellow solid |
| insoluble | |
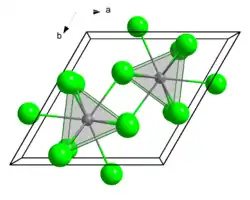
| Structure | |
| hexagonal, UCl3 structure[1] | |
| P63/m, No. 176[2] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |

Curium hydroxide in bottom of microcentrifuge cone, fall 1947
Curium hydroxide is anhydrous colorless[2] or light yellow[5] amorphous gelatinous solid that is insoluble to water.[1] Due to self-irradiation the crystal structure of 244Cm(OH)3 decomposes within one day, while for americium hydroxide 241Am(OH)3 same process takes 4-6 months.[2]
See also
References
- Macintyre, Jane E. (1992). Dictionary of Inorganic Compounds. CRC Press. p. 3046. ISBN 978-0-412-30120-9.
- Krivovichev, Sergei; Burns, Peter; Tananaev, Ivan (2006). Structural Chemistry of Inorganic Actinide Compounds. Elsevier. p. 68. ISBN 978-0-08-046791-7.
- Seaborg, Glenn T. (1963). Man-Made Transuranium Elements. Prentice-Hall.
- "WebElements Periodic Table: Curium". webelements.com. Retrieved January 20, 2019.
- Koch, Günter (1972). Transurane Teil C: Die Verbindungen. Gmelins Handbuch (in German). Springer-Verlag. p. 35. ISBN 978-3-662-11547-3.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.