Diarylide pigment
Diarylide pigments are organic compounds that are used as pigments in inks and related materials. They often are yellow or yellow-green. To some extent, these organic compounds have displaced cadmium sulfide from the market. They exist as yellow powders of low solubility in water.[1]
Production
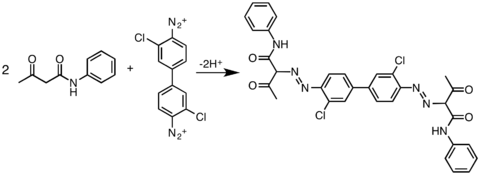
The formation of these pigments involves the reaction of diazotized aromatic diamines (derivatives of benzidine) with coupling components, typically derivatives of acetoacetanilide. By varying both of these components, a wide variety of pigments have been produced.[2] A related family of organic pigments are the simpler arylides, which arise from the coupling of monodiazonium salts with the same coupling partners.
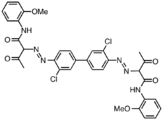
The molecular structures of these molecules is more conjugated than represented in the images shown below.[3] The pigments' colors can range from yellow to green. One of the most common diarylide yellow pigments is Pigment Yellow 12. Its CAS number is . Other diarylide yellow pigments include ‘’’yellow 13’’’ , ‘’’yellow 17’’’ and ‘’’yellow 83’’’ . These pigments share the same backbone and vary in color depending on substitution of the benzene rings. Worldwide production of color organic pigments was estimated to be about 250,000 metric tons (t) in 2006, with about 25%, or 62,500 t, being diarylide yellows.[4]
- Diarylide pigments
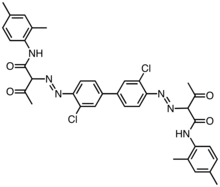

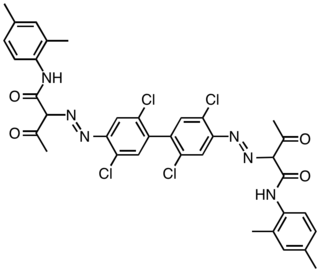
 Pigment Yellow 17
Pigment Yellow 17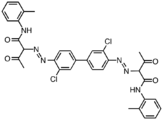 Pigment Yellow 14
Pigment Yellow 14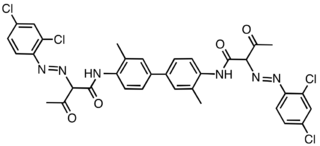
Uses
The diarylide yellows are the most common yellow pigments used in printing as well as a wide variety of other applications. Due to their stability, diarylide yellows are used in inks, coatings, and as plastic colorants. The pigment is insoluble. It is a standard pigment used in printing ink and packaging industry. The diarylide yellow pigment Yellow 12 is one of the three main colored pigments used in the four color process of color printing. As such, its use is ubiquitous in printing both in commercial applications and in home color printers, as well as in textile printing.
Toxicity and hazards
Diarylide yellow pigments are considered to be non-toxic. There is, however, evidence that these pigments degrade when exposed to temperatures above 200 °C to release 3,3'-dichlorobenzidine, a carcinogen that is listed in the U.S. EPA’s Toxics Release Inventory. 3,3’-Dichlorobenzidine is structurally similar to one of the polychlorinated biphenyl congeners, PCB 11 (3,3’-dichlorobiphenyl), and some evidence indicates that the use of diarylide yellow pigments introduces PCB 11 to the environment.[5][6]
See also
- List of colours
References
- K. Hunger. W. Herbst "Pigments, Organic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2012. doi:10.1002/14356007.a20_371
- Lewis, D. M., Modern colorants synthesis and structure. Advances in color chemistry series, Vol. 3. Edited by A. T. Peters & H. S. Freeman, Blackie Academic & Professional, Glasgow, 1995, xii + 245 pp. ISBN 0 7514 0209 5. Journal of Chemical Technology & Biotechnology 1996, 65, (2), 207-208.
- Barrow, M. J.; Christie, R. M.; Monteith, J. E., The crystal and molecular structures of three diarylide yellow pigments, C. I. Pigments Yellow 13, 14 and 63. Dyes and Pigments 2002, 55, (2-3), 79-89.
- Savastano, D., The pigment report: although 2006 was a year of improvement, pigment manufacturers are coping with a wide variety of challenges, including raw material pricing and supply issues and overcapacity. Ink world 2007, March.
- Rodenburg, L. A.; Guo, J.; Du, S.; Cavallo, G. J., Evidence for Unique and Ubiquitous Environmental Sources of 3,3'-Dichlorobiphenyl (PCB 11). Environmental Science & Technology 2009, 44, (8), 2816-2821.
- http://ehp.niehs.nih.gov/121-a86/
