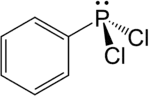
Dichlorophenylphosphine
Dichlorophenylphosphine is an organophosphorus compound with the formula C6H5PCl2. This colourless viscous liquid is commonly used in the synthesis of phosphine ligands.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Phenylphosphonous dichloride | |
| Other names
Dichlorophenylphosphane Phenylphosphorus dichloride | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.010.388 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 2798 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H5Cl2P | |
| Molar mass | 178.98 g·mol−1 |
| Appearance | colorless liquid |
| Odor | acrid, pungent |
| Density | 1.3190 g/mL |
| Melting point | −51 °C (−60 °F; 222 K) |
| Boiling point | 222 °C (432 °F; 495 K) |
| insoluble | |
| Solubility | miscible in benzene, CS2, chloroform |
Refractive index (nD) |
1.6030 |
| Hazards | |
| Safety data sheet | Fisher MSDS |
| GHS pictograms |    |
| GHS Signal word | Danger |
| H290, H301, H302, H314, H318, H335 | |
| P234, P260, P261, P264, P270, P271, P280, P301+310, P301+312, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P312, P321, P330, P363, P390, P403+233, P404, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 101 °C (214 °F; 374 K) |
| 159 °C (318 °F; 432 K) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
200 mg/kg (oral, rat) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dichlorophenylphosphine is commercially available. It may be prepared by an electrophilic substitution of benzene by phosphorus trichloride, catalyzed by aluminium chloride.[1] The compound is an intermediate for the synthesis of other chemicals for instance dimethylphenylphosphine: DOI:
- C6H5PCl2 + 2 CH3MgI → C6H5P(CH3)2 + 2 MgICl
In the McCormack reaction dichlorophenylphosphine adds dienes to give the chlorophospholenium ring.[2]
References
- B. Buchner, L. B. Lockhart, Jr. (1951). "Phenyldichlorophosphine". 31: 88. doi:10.15227/orgsyn.031.0088. Cite journal requires
|journal=(help)CS1 maint: multiple names: authors list (link) - W. B. McCormack (1963). "3-Methyl-1-Phenylphospholene oxide". Org. Synth. 43: 73. doi:10.15227/orgsyn.043.0073.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.

