Dichlorotetrakis(dimethylsulfoxide)ruthenium(II)
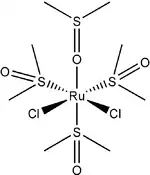
Dichlorotetrakis(dimethyl sulfoxide) ruthenium(II) describes coordination compounds with the formula RuCl2(dmso)4, where DMSO is dimethylsulfoxide. Both cis and trans isomers are known, but the cis isomer is more common. The cis isomer (pictured) is a yellow, air-stable solid that is soluble in some organic solvents. These compounds have attracted attention as possible anti-cancer drugs.
| |||
4_(Strem).jpg.webp) | |||
| Names | |||
|---|---|---|---|
| Systematic IUPAC name
Ruthenium, dichlorotetrakis(sulfinylbis(methane))- (9CI) | |||
| Other names
Tetrakis(dimethylsulfoxide)dichlororuthenium(II), Dichlorotetrakis(methylsulfoxide)ruthenium, Dichlorotetrakis(sulfinylbis(methane))ruthenium | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
PubChem CID |
|||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C8H24Cl2O4RuS4 | |||
| Molar mass | 484.51 g/mol | ||
| Appearance | Various shades of yellow crystals | ||
| Miscible in water | |||
| Solubility | Nitromethane, chloroform, dichloromethane | ||
| Structure | |||
| Octahedral coordinate | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Structure and synthesis
The cis isomer illustrates linkage isomerism for the DMSO ligand.[1] One of the two dmso ligands that are cis to both chloride ligands is O-bonded while the other three dmso ligands are S-bonded. In the trans isomer, which is also yellow, all four dmso ligands are S-bonded. The cis isomer is formed thermally, and the trans isomer is obtained by UV-irradiation of the cis isomer.[2]
ruthenium(II)-from-xtal-2008-3D-balls.png.webp) | ruthenium(II)-from-xtal-1990-3D-balls.png.webp) |
The complexes were first prepared by heating DMSO solutions of ruthenium trichloride under hydrogen atmosphere.[3] An alternative procedure has been developed which avoids hydrogen gas.[2][4]
Potential applications
RuCl2(dmso)4 was identified as a potential anticancer agent in the early 1980s.[5] Continued research[6][7] has led to the development of several related dmso-containing ruthenium compounds, some of which have undergone early-stage clinical trials.[8]
References
- Enzo Alessio (2004). "Synthesis and reactivity of Ru-, Os-, Rh-, and Ir-halide-sulfoxide compounds". Chem. Rev. 104 (9): 4203–4242. doi:10.1021/cr0307291.
- I. Bratsos; E. Alessio (2010). "Ruthenium(II) Chloro Complexes of dimethylsulfoxide". Inorganic Syntheses. 35: 148–152. doi:10.1002/9780470651568.ch8.
- B. R. James; E. Ochiai; G.I. Rempel (1971). "Ruthenium (II) halide dimethylsulphoxide complexes from hydrogenation reactions". Inorganic and Nuclear Chemistry Letters. 7 (8): 781–784. doi:10.1016/0020-1650(71)80091-0.
- Nagy, E. M.; Pettenuzzo, A. (2012). "Ruthenium(II/III)-Based Compounds with Encouraging Antiproliferative Activity against Non-small-Cell Lung Cancer". Chemistry: A European Journal. 18: 14464–14472. doi:10.1002/chem.201202171.
- Sava, Gianni; Giraldi, Tullio; Mestroni, Giovanni; Zassinovich, Grazia (July 1983). "Antitumor effects of rhodium(I), iridium(I) and ruthenium(II) complexes in comparison with cis-dichlorodiammino platinum(II) in mice bearing Lewis lung carcinoma". Chemico-Biological Interactions. 45 (1): 1–6. doi:10.1016/0009-2797(83)90037-6.
- Coluccia, Mauro; Sava, Gianni; Loseto, Francesco; Nassi, Anna; Boccarelli, Angela; Giordano, Domenico; Alessio, Enzo; Mestroni, Giovanni (January 1993). "Anti-leukaemic action of RuCl2(DMSO)4 isomers and prevention of brain involvement on P388 leukaemia and on subline". European Journal of Cancer. 29 (13): 1873–1879. doi:10.1016/0959-8049(93)90541-M.
- Bratsos, I; Serli, B; Zangranko, E; Katsaros, N; Alessio, E. (2007). "Replacement of chlorides with dicarboxylate ligands in anticancer active Ru(II)-DMSO compounds: A new strategy that might lead to improved activity". Inorg. Chem. 46 (3): 975–992. doi:10.1021/ic0613964. PMID 17257042.
- Enzo Alessio, Bentham Science Publisher; Giovanni Mestroni, Bentham Science Publisher; Alberta Bergamo, Bentham Science Publisher; Gianni Sava, Bentham Science Publisher (1 November 2004). "Ruthenium Antimetastatic Agents". Current Topics in Medicinal Chemistry. 4 (15): 1525–1535. doi:10.2174/1568026043387421.