Diethyl carbonate
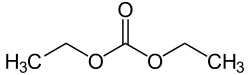
Diethyl carbonate (sometimes abbreviated DEC) is an ester of carbonic acid and ethanol with the formula OC(OCH2CH3)2. At room temperature (25 °C) diethyl carbonate is a clear liquid with a low flash point.
 | |
| Names | |
|---|---|
| Other names
carbonic ether; ethyl carbonate; Eufin[1] | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.011 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 2366 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H10O3 | |
| Molar mass | 118.132 g·mol−1 |
| Appearance | Clear colorless liquid |
| Density | 0.975 g/cm3 |
| Melting point | −74.3[2] °C (−101.7 °F; 198.8 K) |
| Boiling point | 126 to 128 °C (259 to 262 °F; 399 to 401 K) |
| Insoluble | |
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Warning |
| H226, H315, H319, H335 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P302+352, P303+361+353, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P370+378, P403+233, P403+235, P405, P501 | |
| Flash point | 33 °C (91 °F; 306 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Diethyl carbonate is used as a solvent such as in erythromycin intramuscular injections.[3][4] [5]It can be used as a component of electrolytes in lithium batteries. It has been proposed as a fuel additive to support cleaner diesel fuel combustion because its high boiling point might reduce blended fuels' volatility, minimizing vapor buildup in warm weather that can block fuel lines.[6]
Production
It can be made by reacting phosgene with ethanol, producing hydrogen chloride as a byproduct. Because chloroform can react with oxygen to form phosgene, chloroform can be stabilized for storage by adding 1 part (by mass) of ethanol to 100 parts (by mass) of chloroform, so that any phosgene that forms is converted into diethyl carbonate.
See also
References
- "DIETHYL CARBONATE". Retrieved 2010-02-01.
- Ding, Michael (2001). "Liquid/Solid Phase Diagrams of Binary Carbonates for Lithium Batteries". Journal of the Electrochemical Society. 148: A299–A304. doi:10.1149/1.1353568.
- Anderson, Robert C.; Harris, Paul N.; Chen, K. K. (1955). "Further toxicological studies with ilotycin® (Erythromycin, Lilly)". Journal of the American Pharmaceutical Association. 44 (4): 199–204. doi:10.1002/jps.3030440404. ISSN 1930-2304.
- , "9-Dihydro-11,12-ketal derivatives of erythromycin A and epi-erythromycin A", issued 1982-03-01
- , "3",4"-Oxyallylene erythromycin and oleandomycin, composition and method of use", issued 1982-03-01
- Walter, K. Scientists Discover Method for Cleaner Fossil Fuel. MR&D Magazine. 09/18/2017 - 3:16pm
