Diffusive gradients in thin films
The diffusive gradients in thin films (DGT) technique is an environmental chemistry technique for the detection of elements and compounds in aqueous environments, including natural waters,[1] sediments[2] and soils.[3] It is well suited to in situ detection of bioavailable toxic trace metal contaminants.[4][5][6] The technique involves using a specially-designed passive sampler that houses a binding gel, diffusive gel and membrane filter. The element or compound passes through the membrane filter and diffusive gel and is assimilated by the binding gel in a rate-controlled manner. Post-deployment analysis of the binding gel can be used to determine the time-weighted-average bulk solution concentration of the element or compound via a simple equation.
History
The DGT technique was developed in 1994 by Hao Zhang and William Davison at the Lancaster Environment Centre of Lancaster University in the United Kingdom. The technique was first used to detect metal cations in marine environments using Chelex 100 as the binding agent. Further characterisation of DGT, including the results of field deployments in the Menai Strait and the North Atlantic Ocean, was published in 1995.[7] The technique was first tested in soils in 1998, with results demonstrating that kinetics of dissociation of labile species in the porewater (soil solution) could be determined via DGT.[8] Since then, the DGT technique has been modified and expanded to include a significant number of elements and compounds, including cationic metals,[7] phosphate and other oxyanions (V, CrVI, As, Se, Mo, Sb, W),[2][9][10][11][12][13] antibiotics,[14] bisphenols,[15] and nanoparticles,[16] and has even been modified for the geochemical exploration of gold.[17] In 2010, EasySensor DGT has ben under development by Prof. Shiming Ding and co-workers. Due to their high capacity, wide-range of tolerance. long storage periods and simple operation, EasySensor DGT products can be widely used on complex environmental medium with high pollution, high nutrition and high pH, which meets the monitoring requirements of most soils, water bodies and sediments. (www.global-easysensor.com)
The DGT device

The DGT device is made of plastic, and comprises a piston and a tight-fitting, circular cap with an opening (DGT window). A binding gel, diffusive gel and filter membrane are stacked onto the piston, and the cap is placed over the assembly. The dimensions of the device normally ensure that the two gels and filter membrane are well-sealed when the cap is put on.[4]:4.2.3 Dimensions of the layers vary depending on features of the environment, such as the flow rate of water being sampled;[4]:4.2.1 an example is an approximately 2 cm device diameter containing a 1mm gel layer.[18] Different from DGTresearch. the EasySensor DGT device employs two new holder configurations, double-mode and flat types. (link: chrome-extension://ibllepbpahcoppkjjllbabhnigcbffpi/https://hal.archives-ouvertes.fr/hal-02142638/document).
The cavity type is a new model for dry soil detection developed by Ding et al., (2016). The soil was loaded into the cavity until filled and leveled. Before the appearance of the cavity type, the piston type was commonly used by pressing the DGT onto the surface of the soil layer. This method could easily cause the change of the density of the soil and affect the free diffusion of the target ions to the DGT device, which resulting in the analysis error. When the soil is placed using the cavity mode, the soil relies on gravity to contact the exposed surface of the DGT device, thus avoiding the errors caused by manual pressing.
Principles of operation
Deployment

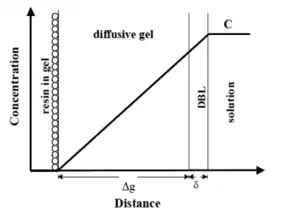
DGT devices can be directly deployed in aqueous environmental media, including natural waters, sediments, and soils.[1] In fast-flowing waters, the DGT device's face should be perpendicular to the direction of flow, in order to ensure the diffusive boundary layer (DBL) is not affected by laminar flow. In slow-flowing or stagnant waters such as in ponds or groundwater, deployment of DGT devices with different thicknesses of diffusive gel can allow for the determination of the DBL and a more accurate determination of bulk concentration.[4]:4.2.1[19] Modifications to the diffusive gel (e.g. increasing or decreasing the thickness) can also be undertaken to ensure low detection limits.[20]
Analysis of binding gels and chemical imaging
After the DGT devices/probes have been retrieved, the binding gels can be eluted using methods that depend on the target analyte and the DGT binding gel (for example, nitric acid can be used to elute most metal cations from Chelex-100 gels).[4]:4.2.1 NaOH can be used to elute most oxyanions from Zr-Oxide(Ding et al., 2010, 2011,2016; Sun et al.,2014).The eluent can then be quantitatively analysed via a range of analytical techniques, including but not limited to: ICP-MS, GFAAS[4]:4.2.1 ICP-OES, AAS,[18] UV-Vis spectroscopy or computer imaging densitometry.[21] For chemical imaging and to obtain two-dimensional (2D) sub-mm high resolution distribution of analytes in heterogenous environments, such as sediments and the rhizosphere, the retrieved gel strips can be analyzed by PIXE or LA-ICP-MS after gel drying.[11][22][23][24][25]
The DGT equation
DGT is based on the application of Fick's law.[18] Once the mass of an analyte has been determined, the time-averaged concentration of the analyte in the bulk, , can be determined by application of the following equation:
where is the mass of the analyte on the resin, is the thickness of the diffusive layer and filter membrane together, is the diffusion coefficient of the analyte, is the deployment time, and is the area of the DGT window.[4]:Eq.2 More elaborate analysis techniques may be required in cases where the ionic strength of the water is low and where significant organic matter is present.[26]
See also
References
- Chaudhary, Meenakshi; Quanz, Meaghan; Williams, Jim; Maltby, Ella; Oakes, Ken; Spooner, Ian; Walker, Tony R. (September 2020). "Assessment of metal(loid) concentrations using Diffusive Gradient Thin (DGT) films in marine, freshwater and wetland aquatic ecosystems impacted by industrial effluents". Case Studies in Chemical and Environmental Engineering: 100041. doi:10.1016/j.cscee.2020.100041.
- Zhang, Hao; Davison, William; Gadi, Ranu; Kobayashi, Takahiro (August 1998). "In situ measurement of dissolved phosphorus in natural waters using DGT". Analytica Chimica Acta. 370 (1): 29–38. doi:10.1016/S0003-2670(98)00250-5.
- Wilkins, C. (21 October 1983). "Hyphenated techniques for analysis of complex organic mixtures". Science. 222 (4621): 291–296. Bibcode:1983Sci...222..291W. doi:10.1126/science.6353577. PMID 6353577.
- "Diffusive Gradients in Thin-films (DGT): A Technique for Determining Bioavailable Metal Concentrations" (PDF). International Network for Acid Prevention. March 2002. Retrieved 23 April 2015.
- Strivens, Jonathan; Hayman, Nicholas; Johnston, Robert; Rosen, Gunther (May 2019). "Effects of Dissolved Organic Carbon on Copper Toxicity to Embryos of Mytilus galloprovincialis as Measured by Diffusive Gradient in Thin Films". Environmental Toxicology and Chemistry. 38 (5): 1029–1034. doi:10.1002/etc.4404. PMID 30840314.
- Strivens, Jonathan; Hayman, Nicholas; Rosen, Gunther; Myers‐Pigg, Allison (April 2020). "Toward Validation of Toxicological Interpretation of Diffusive Gradients in Thin Films in Marine Waters Impacted by Copper". Environmental Toxicology and Chemistry. 39 (4): 873–881. doi:10.1002/etc.4673. PMID 32004383.
- Zhang, Hao; Davison, William (October 1995). "Performance Characteristics of Diffusion Gradients in Thin Films for the in Situ Measurement of Trace Metals in Aqueous Solution". Analytical Chemistry. 67 (19): 3391–3400. doi:10.1021/ac00115a005.
- Harper, Michael P; Davison, William; Zhang, Hao; Tych, Wlodek (August 1998). "Kinetics of metal exchange between solids and solutions in sediments and soils interpreted from DGT measured fluxes". Geochimica et Cosmochimica Acta. 62 (16): 2757–2770. Bibcode:1998GeCoA..62.2757H. doi:10.1016/S0016-7037(98)00186-0.
- Santner, Jakob; Prohaska, Thomas; Luo, Jun; Zhang, Hao (15 September 2010). "Ferrihydrite Containing Gel for Chemical Imaging of Labile Phosphate Species in Sediments and Soils Using Diffusive Gradients in Thin Films". Analytical Chemistry. 82 (18): 7668–7674. doi:10.1021/ac101450j. PMC 3432420. PMID 20735010.
- Luo, Jun; Zhang, Hao; Santner, Jakob; Davison, William (November 2010). "Performance Characteristics of Diffusive Gradients in Thin Films Equipped with a Binding Gel Layer Containing Precipitated Ferrihydrite for Measuring Arsenic(V), Selenium(VI), Vanadium(V), and Antimony(V)". Analytical Chemistry. 82 (21): 8903–8909. doi:10.1021/ac101676w. PMID 20936784.
- Guan, Dong-Xing; Williams, Paul N.; Luo, Jun; Zheng, Jian-Lun; Xu, Hua-Cheng; Cai, Chao; Ma, Lena Q. (17 March 2015). "Novel Precipitated Zirconia-Based DGT Technique for High-Resolution Imaging of Oxyanions in Waters and Sediments". Environmental Science & Technology. 49 (6): 3653–3661. Bibcode:2015EnST...49.3653G. doi:10.1021/es505424m. PMID 25655234.
- Stockdale, Anthony; Davison, William; Zhang, Hao (2010). "2D simultaneous measurement of the oxyanions of P, V, As, Mo, Sb, W and U" (PDF). Journal of Environmental Monitoring. 12 (4): 981–4. doi:10.1039/b925627j. PMID 20383381.
- Pan, Yue; Guan, Dong-Xing; Zhao, Di; Luo, Jun; Zhang, Hao; Davison, William; Ma, Lena Q. (15 December 2015). "Novel Speciation Method Based on Diffusive Gradients in Thin-Films for in Situ Measurement of Cr VI in Aquatic Systems". Environmental Science & Technology. 49 (24): 14267–14273. Bibcode:2015EnST...4914267P. doi:10.1021/acs.est.5b03742. PMID 26535488.
- Chen, Chang-Er; Zhang, Hao; Jones, Kevin C. (2012). "A novel passive water sampler for in situ sampling of antibiotics". Journal of Environmental Monitoring. 14 (6): 1523–30. doi:10.1039/c2em30091e. PMID 22538362.
- Zheng, Jian-Lun; Guan, Dong-Xing; Luo, Jun; Zhang, Hao; Davison, William; Cui, Xin-Yi; Wang, Lian-Hong; Ma, Lena Q. (6 January 2015). "Activated Charcoal Based Diffusive Gradients in Thin Films for in Situ Monitoring of Bisphenols in Waters". Analytical Chemistry. 87 (1): 801–807. doi:10.1021/ac503814j. PMID 25412473.
- Pouran, Hamid M.; Martin, Francis L.; Zhang, Hao (17 June 2014). "Measurement of ZnO Nanoparticles Using Diffusive Gradients in Thin Films: Binding and Diffusional Characteristics". Analytical Chemistry. 86 (12): 5906–5913. doi:10.1021/ac500730s. hdl:2436/621804. PMID 24831848.
- Lucas, Andrew; Rate, Andrew; Zhang, Hao; Salmon, S. Ursula; Radford, Nigel (21 August 2012). "Development of the Diffusive Gradients in Thin Films Technique for the Measurement of Labile Gold in Natural Waters". Analytical Chemistry. 84 (16): 6994–7000. doi:10.1021/ac301003g. PMID 22812590.
- Thomas, P. (1 July 2009). "Metals pollution tracing in the sewerage network using the diffusive gradients in thin films technique". Water Science and Technology. 60 (1): 65–70. doi:10.2166/wst.2009.287. PMID 19587403.
- Warnken, Kent W.; Zhang, Hao; Davison, William (June 2006). "Accuracy of the Diffusive Gradients in Thin-Films Technique: Diffusive Boundary Layer and Effective Sampling Area Considerations". Analytical Chemistry. 78 (11): 3780–3787. doi:10.1021/ac060139d. PMID 16737237.
- Lucas, Andrew R.; Reid, Nathan; Salmon, S. Ursula; Rate, Andrew W. (21 October 2014). "Quantitative Assessment of the Distribution of Dissolved Au, As and Sb in Groundwater Using the Diffusive Gradients in Thin Films Technique". Environmental Science & Technology. 48 (20): 12141–12149. Bibcode:2014EnST...4812141L. doi:10.1021/es502468d. PMID 25252140.
- McGifford, RW; Seen, AJ; Haddad, PR (3 March 2010). "Direct colorimetric detection of copper(II) ions in sampling using diffusive gradients in thin-films". Analytica Chimica Acta. 662 (1): 44–50. doi:10.1016/j.aca.2009.12.041. PMID 20152264.
- Davison, W.; Fones, G. R.; Grime, G. W. (June 1997). "Dissolved metals in surface sediment and a microbial mat at 100-μm resolution". Nature. 387 (6636): 885–888. Bibcode:1997Natur.387..885D. doi:10.1038/43147. S2CID 4261454.
- Warnken, Kent W.; Zhang, Hao; Davison, William (October 2004). "Analysis of Polyacrylamide Gels for Trace Metals Using Diffusive Gradients in Thin Films and Laser Ablation Inductively Coupled Plasma Mass Spectrometry". Analytical Chemistry. 76 (20): 6077–6084. doi:10.1021/ac0400358. PMID 15481956.
- Williams, Paul N.; Santner, Jakob; Larsen, Morten; Lehto, Niklas J.; Oburger, Eva; Wenzel, Walter; Glud, Ronnie N.; Davison, William; Zhang, Hao (5 August 2014). "Localized Flux Maxima of Arsenic, Lead, and Iron around Root Apices in Flooded Lowland Rice". Environmental Science & Technology. 48 (15): 8498–8506. Bibcode:2014EnST...48.8498W. doi:10.1021/es501127k. PMC 4124062. PMID 24967508.
- Hoefer, Christoph; Santner, Jakob; Puschenreiter, Markus; Wenzel, Walter W. (7 April 2015). "Localized Metal Solubilization in the Rhizosphere of Salix smithiana upon Sulfur Application". Environmental Science & Technology. 49 (7): 4522–4529. Bibcode:2015EnST...49.4522H. doi:10.1021/es505758j. PMC 4394708. PMID 25782052.
- Yabuki, Lauren Nozomi Marques; Colaço, Camila Destro; Menegário, Amauri Antonio; Domingos, Roberto Naves; Kiang, Chang Hung; Pascoaloto, Domitila (February 2014). "Evaluation of diffusive gradients in thin films technique (DGT) for measuring Al, Cd, Co, Cu, Mn, Ni, and Zn in Amazonian rivers". Environmental Monitoring and Assessment. 186 (2): 961–969. doi:10.1007/s10661-013-3430-x. PMID 24052239. S2CID 9781883.
Ding SM, Xu D, Sun Q, et al. Measurement of dissolved reactive phosphorus using the diffusive gradients in thin films technique with a high-capacity binding phase. Environmental Science &Technology, 2010, 44(21): 8169-8174. Link: https://pubs.acs.org/doi/10.1021/es1020873
Ding SM, Jia F, Xu D, et al. High-resolution, two-dimensional measurement of dissolved reactive phosphorus in sediments using the diffusive gradients in thin films technique in combination with a routine procedure. Environmental Science & Technology, 2011, 45(22), 9680-9686. Link: https://pubs.acs.org/doi/10.1021/es202785p
Ding S M, Sun Q, Xu D, et al. High-resolution simultaneous measurements of dissolved reactive phosphorus and dissolved sulfide: the first observation of their simultaneous release in sediments. Environmental Science & Technology, 2012, 46(15): 8297-8304. Link: https://pubs.acs.org/doi/10.1021/es301134h
Ding SM, Wang Y, Xu D, et al. Gel-Based Coloration Technique for the Submillimeter-Scale Imaging of Labile Phosphorus in Sediments and Soils with Diffusive Gradients in Thin Films. Environmental Science & Technology, 2013, 47(14): 7821-7829. Link: https://pubs.acs.org/doi/10.1021/es400192j
Ding SM, Xu D, Wang YP, et al. Simultaneous measurements of eight oxyanions using high-capacity diffusive gradients in thin films (Zr-oxide DGT) with a high-efficiency elution procedure. Environmental Science & Technology, 2016, 50(14): 7572-7580. Link: https://pubs.acs.org/doi/10.1021/acs.est.6b00206
Ding SM, Wang Y, Zhang LP, et al. New holder configurations for use in the diffusive gradients in thin films (DGT) technique. RSC Advances, 2016, 6(91): 88143-88156. Link: https://pubs.rsc.org/en/content/articlelanding/2016/RA/C6RA19677B#!divAbstract
Xu D, Chen Y F, Ding S M, et al. Diffusive gradients in thin films technique equipped with a mixed binding gel for simultaneous measurements of dissolved reactive phosphorus and dissolved iron. Environmental Science & Technology, 2013, 47(18): 10477-10484. Link: https://pubs.acs.org/doi/abs/10.1021/es401822x