Eriodictyol
Eriodictyol is a bitter-masking flavanone, a flavonoid extracted from yerba santa (Eriodictyon californicum), a plant native to North America.[1] Eriodictyol is one of the four flavanones identified in this plant as having taste-modifying properties, the other three being homoeriodictyol, its sodium salt, and sterubin.[2]
 | |
| Names | |
|---|---|
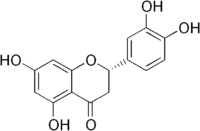
| IUPAC name
(2S)-2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-chromanone | |
| Other names
Eriodictiol | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.008.198 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H12O6 | |
| Molar mass | 288.255 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Eriodictyol was also found in the twigs of Millettia duchesnei,[3] in Eupatorium arnottianum,[4] and its glycosides (eriocitrin) in lemons and rose hips (Rosa canina).[5]
References
- Patricia Kaminski and Richard Katz. Yerba Santa Eriodictyon californicum. Flower Essence Society.
- Ley JP, Krammer G, Reinders G, Gatfield IL, Bertram HJ (July 2005). "Evaluation of bitter masking flavanones from Herba Santa (Eriodictyon californicum (H. and A.) Torr., Hydrophyllaceae)". J. Agric. Food Chem. 53 (15): 6061–6. doi:10.1021/jf0505170. PMID 16028996.
- Ngandeu F, Bezabih M, Ngamga D, et al. (January 2008). "Rotenoid derivatives and other constituents of the twigs of Millettia duchesnei". Phytochemistry. 69 (1): 258–63. doi:10.1016/j.phytochem.2007.05.038. PMID 17640692.
- Clavin M, Gorzalczany S, Macho A, et al. (July 2007). "Anti-inflammatory activity of flavonoids from Eupatorium arnottianum". J Ethnopharmacol. 112 (3): 585–9. doi:10.1016/j.jep.2007.04.007. PMID 17570627.
- Hvattum E (2002). "Determination of phenolic compounds in rose hip (Rosa canina) using liquid chromatography coupled to electrospray ionisation tandem mass spectrometry and diode-array detection". Rapid Commun. Mass Spectrom. 16 (7): 655–62. doi:10.1002/rcm.622. PMID 11921243.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
