Eukaryote hybrid genome
Eukaryote hybrid genomes result from interspecific hybridization, where closely related species mate and produce offspring with admixed genomes. The advent of large-scale genomic sequencing has shown that hybridization is common, and that it may represent an important source of novel variation. Although most interspecific hybrids are sterile or less fit than their parents, some may survive and reproduce, enabling the transfer of adaptive variants across the species boundary, and even result in the formation of novel evolutionary lineages. There are two main variants of hybrid species genomes: allopolyploid, which have one full chromosome set from each parent species, and homoploid, which are a mosaic of the parent species genomes with no increase in chromosome number. The establishment of hybrid species requires the development of reproductive isolation against parental species. Allopolyploid species often have strong intrinsic reproductive barriers due to differences in chromosome number, and homoploid hybrids can become reproductively isolated from the parent species through assortment of genetic incompatibilities. However, both types of hybrids can become further reproductively isolated, gaining extrinsic isolation barriers, by exploiting novel ecological niches, relative to their parents. Hybrids represent the merging of divergent genomes and thus face problems arising from incompatible combinations of genes. Thus hybrid genomes are highly dynamic and may undergo rapid evolutionary change, including genome stabilization in which selection against incompatible combinations results in fixation of compatible ancestry block combinations within the hybrid species. The potential for rapid adaptation or speciation makes hybrid genomes a particularly exciting subject of in evolutionary biology. The article summarizes how introgressed alleles or hybrid species can establish and how the resulting hybrid genomes evolve.
| Glossary | |
|---|---|
|
Background
Genetic exchange between species can impede the evolution of biodiversity because gene flow between diverging species counteracts their differentiation and hybridization between recently diverged species can lead to loss of genetic adaptations or species fusion.[1] Traditionally, zoologists have viewed interspecific hybridization as maladaptive behaviour[2] which can result in breaking up co-adapted gene complexes.[3] In contrast, plant biologists recognized early on that hybridization can sometimes be an important evolutionary force, contributing to increasing biodiversity.[4][5] Recently, evidence has been accumulating showing that hybridization is also an important evolutionary process in animals.[1][6][7] Interspecific hybridization can enrich the genetic diversity of introgressed taxon, lead to introgression of beneficial genetic variation or even generate new hybrid species.[1] Hybridization is now also known to contribute to the evolutionary potential in several textbook examples of adaptive radiation, including the Geospiza Galapagos finches,[8] African cichlid fishes,[9] Heliconius butterflies[10][11][12] and Hawaiian Madiinae tarweeds and silverswords.[13] This article reviews the evolutionary outcomes of interspecific hybridization and the properties of genomes of hybrid genomes. Many of the discussed topics also apply to hybridization between different subspecies or populations of the same species, but this article focuses on interspecific hybridization (referred to as hybridization in this review).
Evolutionary outcomes
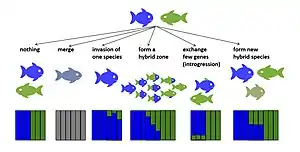
There are several potential evolutionary outcomes of hybridization. If early generation hybrids are not viable or sterile, hybridization may reduce the reproductive success of the parent species.[14][15] This could potentially lead to reinforcement, selection to strengthen premating isolation[16] or if the species fail to evolve premating isolation, it could increase their extinction risk due to wasted reproductive effort.[14] If the fitness of early generation hybrids is non-zero and that of some later generation hybrids is as high or even higher than the fitness of one or both parent taxa, hybrids may displace the parent taxa and the hybridizing taxa may fuse (speciation reversal[17][18]). If the fitness of early generation hybrids is reduced but non-zero, hybrid zones may emerge in the contact zone of the taxa.[19] If hybrids are fertile, hybridization may contribute novel variation through rare hybrids backcrosssing with parental species. Such introgressive hybridization may enable neutral or selectively beneficial alleles to be transferred across species boundaries even in species pairs that remain distinct despite occasional gene flow.[20][21] Hybrid fitness may vary with divergence time between the hybridizing taxa. This pattern has been shown for a variety of taxa including Drosophila,[22] birds[23] and fish.[24] Hybrid fitness may also differ with cross direction,[25] between first generation and later generation hybrids,[26] and among individuals within generations of the same cross-type.[27][28] In some cases hybrids may evolve into new hybrid species with reproductive isolation to both parent taxa.[29][30] Below is described the evolutionary outcomes of hybridisation that result in persistent hybrid genomes.
Adaptive introgression
When rare hybrids backcross with parent species alleles coding for traits that are beneficial for both parental species can be transferred across species boundaries, even if parent species remain distinct taxa. This process is referred to as adaptive introgression (a somewhat misleading term because backcrossing itself may not be adaptive, but some of the introgressed variants may be beneficial[1]). Simulations suggest that adaptive introgression is possible unless hybrid fitness is substantially reduced,[31][32] or the adaptive loci are tightly linked to deleterious ones.[33] Examples of adaptive traits that have been transferred via introgression include an insecticide resistance gene that was transferred from Anopheles gambiae to A. coluzzii[21] and the red warning wing colouration trait in Heliconius butterflies that is under natural selection from predators which has been introgressed from e.g. H. melpomene to H. timareta[34] and other Heliconius species.[20] In the plant Arabidopsis arenosa some of the alleles conferring adaptation to drought and phytotoxic levels of metal have been introgressed from A. lyrata.[35] Even in humans there is evidence for adaptive introgression of e.g. immunity alleles, skin pigmentation alleles and alleles conferring adaptation to high altitude environments from Neanderthal and Denisovans.[36] If traits important for species recognition or reproductive isolation introgress into a population of another species, the introgressed population may become reproductively isolated against other populations of the same species. Examples of this include Heliconius butterflies, where selective introgression of wing pattern genes between diverged lineages occurs,[37] and wing patterns contribute to reproductive isolation in some species pairs with low (e.g. between H. t. florencia and H. t. linaresi) and intermediate levels (e.g. H. c. galanthus/H. pachinus) of divergence.[38]
Detection and study with genomic tools
Many empirical case studies start with exploratory detection of putative hybrid taxa or individuals with genomic clustering approaches, such as those used in the software STRUCTURE,[39] ADMIXTURE[40] or fineSTRUCTURE.[41] These methods infer a user-specified number of genetic groups from the data and assign each individual to one or a mix of these groups. They can be applied to closely related taxa without having to preassign individuals to taxa and may thus be particularly useful in the study of closely related taxa or species complexes. However, uneven sampling of the parental taxa or different amounts of drift in the included taxa may lead to erroneous conclusions about evidence for hybridization.[42]
If genomic data of multiple species is available, phylogenetic methods may be better suited to identify introgression. Introgressive hybridization leads to gene trees that are discordant from the species tree, whereby introgressed individuals are phylogenetically closer to the source of introgression than to their non-introgressed conspecifics. Such discordant gene trees can also arise by chance through incomplete lineage sorting, particularly if the species compared are still young. Therefore, discordant gene trees are only evidence of introgression if a gene tree produced by excess allele sharing between the hybridizing taxa is strongly overrepresented compared to alternative discordant gene trees. An entire suite of methods have been developed to detect such excess allele sharing between hybridizing taxa, including Patterson’s D statistics or ABBA-BABA tests[43][44][45] or f-statistics.[46][47] Modified versions of these tests can be used to infer introgressed genomic regions,[48] the direction of gene flow[49][50] or the amount of gene flow.[47]
For datasets with a large number of taxa it may be difficult to compute all possible test of hybridization. In such cases, graph construction methods may be better suited.[51][52][53] These methods reconstruct complex phylogenetic models with hybridization that best fit the genetic relationships among the sampled taxa and provide estimates for drift and introgression. Other phylogenetic network methods that account for incomplete lineage sorting and hybridization may also help.[54][55] Methods based on linkage disequilibrium decay or methods inferring ancestry tracts can be used to date recent admixture or introgression events as over time ancestry tracts are continuously broken down by recombination.[52][56][57][58][59] With increasing genome stabilization, individuals should vary less in local ancestry. Levels of genome stabilization can thus be assessed by computing the ancestry proportions (e.g. with fd) in genomic windows and testing if these correlate across individuals. Additionally, if hybridization is still ongoing, ancestry proportions should vary across individuals and in space.
A different approach is to use demographic modelling to find the simplification of the evolutionary history of the studied taxa.[60] Demographic modelling should only be applied to small sets of taxa because with increasing number of taxa model complexity increases and the number of model parameters such as timing, amounts and direction of gene flow, and population sizes and split times can quickly become too high. The fit of the demographic models to the data can be assessed with the site frequency spectrum[61][62] or with summary statistics in an Approximate Bayesian Computation framework.[63] It is also possible to gain more power by combining information from linkage disequilibrium decay patterns and the allele frequency spectrum.[64]
Hybrid species definition
One of the potential evolutionary outcomes of hybridisation is the establishment of a novel, reproductively isolated lineage, i.e., hybrid speciation.[1][29] A hybrid species has an admixed genome and forms stable genetically distinct populations.[29] Some researchers argue that evidence of a hybridization-derived basis for reproductive isolation should be an additional defining criterion for hybrid speciation,[65] but see.[66] This stricter definition includes polyploid hybrid taxa but only encompasses a handful of well studied cases of homoploid hybrid speciation, e.g. Heliconius heurippa,[10][11][12] Passer italiae,[28] and three Helianthus sunflower species[67] because for most suggested examples of homoploid hybrid speciation, the genetic basis of reproductive isolation is still unknown.[65]
Hybrid species can occupy an ecological niche different to those of the parents and may be isolated from the parent species primarily through pre-mating barriers (hybrid speciation with external barriers[68]). Hybrid species may also be reproductively isolated from the parent species through sorting of incompatibilities leading to new combinations of parental alleles that are incompatible with both parent species but compatible within the hybrid taxon (recombinational hybrid speciation).[29] A recombinational hybrid taxon typically also has a substantial proportion of the genome derived from the donor of introgressed material, although variation exists both between taxa and within lineages of hybrid taxa.[69][70]
Homoploid and polyploid hybrid speciation
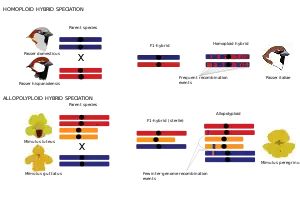
In general, hybrid species can arise from two major types of hybrid speciation, defined by whether the speciation event is associated with genome duplication (polyploidy) or not. Homoploid hybrid speciation Homoploid hybrid speciation is defined as the evolution of a new hybrid species with reproductive isolation to both parent taxa without change of ploidy, i.e. number of chromosome sets.[1] The genomes of homoploid hybrid species are mosaics of the parent genomes as ancestry tracts from the parent species are broken up by recombination.[66][67][71][72][73][74][75] In the case of polyploid hybrid speciation, hybridisation is associated with genome duplication, resulting in an allopolyploid with increased ploidy compared to their parental taxa. In contrast to allopolyploids, autopolyploids are characterised by genome duplication within the same species and are thus not discussed further in the context of this review. Allopolyploid speciation is more common in plants than in animals.[76] Polyploid hybrids can be instantly isolated from their parental species through chromosome number differences.[76]
Reproductive isolation against parent species
Sufficient reproductive isolation from both parental species is required for the successful establishment of a hybrid species.[1][65][77] Reproductive isolation against parent species is harder to achieve for homoploid hybrids where karyotype differences do not contribute to intrinsic isolation. Reproductive isolation between a hybrid species and its parental species can arise from a variety of reproductive barriers either before or after fertilization (prezygotic or postzygotic, respectively), which may themselves be dependent or independent of environmental conditions (extrinsic or intrinsic barriers, respectively).[78] For example, intrinsic postzygotic barriers cause hybrid inviability or sterility regardless of the environment in which they occur, while extrinsic postzygotic barriers result in hybrids of low fitness due to maladaptation to specific environments.[30]
Prezygotic intrinsic and extrinsic differences have also been shown to be important in isolating hybrids from their parent species. In plants, pollinator mediated isolation resulting from changes in floral characteristics may be an important extrinsic prezygotic ecological barrier.[79][80][81][82] Strong extrinsic pre-zygotic has been shown to isolate the hybrid species Senecio eboracensis from its parent species, where hybrids are virtually absent in the wild, although a fraction of hybrid offspring are fertile in lab experiments.[83] Lowe & Abbott conclude that selfing, timing of flowering and characters involved in pollinator attraction likely contribute to this external isolation.[83] Prezygotic mate preference driven isolation generated from intrinsic assortative mating between hybrids has also been reported in several taxa. In African cichlid fish, experimental hybrids displayed combinations of parental traits and preferences which resulted in hybrids predominantly mating with other hybrids.[84] A similar pattern was found in Geospiza Galapagos finches where a specific hybrid song resulted from the transgressive beak morphology,[8] and hybrid Heliconius butterflies preferred the hybrid wing patterning over that of both parent species.[12] Intrinsic differences in habitat use[85] or in phenology[86] may result in some degree of reproductive isolation against parent species if mating is time and habitat-specific. For example the apple host race in Rhagoletis pomonella maggot flies evolved after introgression of diapause related genes from Mexican altiplano flies that allowed a switch from the ancestral host hawthorne to the later flowering apple [87][88] and isolated the two host races via allochronic intrinsic pre-zygotic isolation. In Xiphophorus swordtail fish strong ancestry assortative mating maintained a hybrid genetic cluster separate for 25 generations, but disappeared under manipulated conditions.[89] Hence, prezygotic reproductive barriers to gene flow may be environment dependent.
Postzygotic isolating barriers have also been shown to be important in a variety of hybrid lineages. Work on Helianthus sunflowers has revealed that intrinsic postzygotic can cause reproductive isolation against the parent species. The postzygotic barriers consist in pre-existing structural differences,[73][90] in combination with hybridization induced structural differences.[73] Sorting of incompatibilities between parent species, where one subset of these isolates the hybrid taxon against one parent and a different subset isolates it against the other parent, has resulted in intrinsic postzygotic isolation between the Italian sparrow Passer italiae and its parent species.[28] Simulation studies show that the likelihood of hybrid speciation through this mechanism depends on the divergence time between parent species,[91] the population size of the hybrid species,[92] the nature of selection acting on hybrids, and linkage among incompatibilities to each other and to adaptive variants.[93] Extrinsic ecological barriers against parent species may arise as by-products of ecological differentiation if mating is time and/or habitat specific. Hybrid species have been shown to adapt to novel ecological niches through transgressive phenotypes,[85] or through novel combinations of ecological traits from the parent species,[94] and ecological selection against parent-hybrid cross phenotypes would result in extrinsic postzygotic isolation.
Stabilization
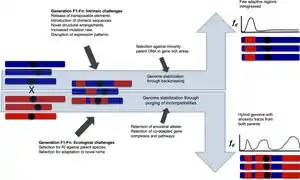
Hybridization can have many different outcomes. Hybrid speciation results in reproductive isolation against both parent species and genomes that evolve independently from those of the parent species. Introgressive hybridization can transfer important novel variants into genomes of a species that remains distinct from the other taxa in spite of occasional gene flow. In this article both types of hybridization-derived genomes are referred to as persistent hybrid genomes. Following initial hybridization, introgression tracts, the genetic blocks inherited from each parent species, are broken down with successive generations and recombination events. Recombination is more frequent in homoploid hybrid genomes than in allopolyploid hybrid genomes. In allopolyploids, recombination can destabilize the karyotype and lead to aberrant meiotic behaviour and reduced fertility, but may also generate novel gene combinations and advantageous phenotypic traits [95] as in homoploid hybrids. Once hybridization between the hybrid taxon and its parent taxa ceases, different ancestry blocks or introgression tracts may become fixed, a process referred to as "genome stabilization".[71] Some introgression tracts are removed by selection against incompatibilities and others are fixed. Theoretical models on hybrid zones suggest that the breakdown of ancestry blocks through recombination is suppressed near genes conferring reproductive isolation due to lower fitness of recombinant hybrids.[96] The strength of the suppression is affected by the form of selection, dominance, and whether the locus was situated on an autosome or sex chromosome.[96] The time to genome stabilization is variable. Fixation of ancestry blocks was found to be rapid in experimental hybrid Helianthus sunflower species genomes,[97] and the genome stabilization of hybrid sunflower species is estimated to take hundreds of generations.[71] In Zymoseptoria fungi genomes were stabilized within ca. 400 generations,[98] whereas in hybrid Xiphophorus swordtail genomes[99] genome stabilization was not achieved until after ca. 2000 and 2500 generations. Few Neanderthal regions have fixed in human genomes during the ca. 2000 generations after hybridization,[100] and segregating incompatibilities are present in the hybrid Italian sparrow approximately 5000 generations after the initial hybridization event.[101]
Given time, genetic drift will eventually stochastically fix blocks derived from the two parent species in finite isolated hybrid populations.[71] Selection against incompatibility loci may accelerate the process of fixation of parental alleles as hybrids that possess alleles that are less likely to cause incompatibility will have higher fitness and favourable alleles will spread in the population. Fixation of recessive weakly deleterious alleles in the parent taxa may, however, also result in hybrids retaining both parental alleles: because hybrids with haplotypes from both parents are not homozygous for any weakly deleterious alleles, they have higher fitness than hybrids with only one parental haplotype. This associative overdominance,[102][103] may slow down the process of fixation of parental alleles through favouring retention of both parental haplotypes. The effect of associative overdominance is strongest in low recombination regions, including inversions.[104] The balance between alleles and allelic combinations providing favourable phenotypic characters and the strength of selection against incompatibilities determine what introgression tracts will be inherited from which parent species upon hybridization.[21][105][106] An insecticide resistance region was retained following a hybridization event in Anopheles coluzzi,[21] suggesting a role for selection in maintaining favourable introgressed regions. The local recombination rate is important for the likelihood of introgression because in the case of widespread incompatibilities, introgressed alleles are more likely to recombine away from incompatibilities in high recombination regions. This pattern has been detected in monkeyflowers Mimulus,[107] in Mus domesticus house mice,[108] in Heliconius butterflies[106] and in Xiphophorus swordtail fish.[69]
Genome-wide incompatibilities have been identified in Xipophorous fish,[109] chimeric genes and mutations of orthologous genes cause incompatibilities in early generation experimental Cyprinidae goldfish - carp hybrids[110] and mito-nuclear incompatibilies are found to have a key role e.g. in Italian sparrows,[75][111] fungus[112] and cyto-nuclear incompatibilities in Mimulus plants.[113] Evidence from altered expression patterns in synthetic hybrids and missing gene combinations in a hybrid species also suggest that DNA-repair[75][110][114] and genes involved in mutagenesis and cancer related pathways[110] may cause incompatibilities in hybrids. Genome formation in hybrid species is shaped by selection against incompatible combinations.[69][99][105]
Altered genome properties
The hybrid origin may affect genome structure and properties. It has been shown to increase mutation rates,[78][115][116] to activate transposable elements,[117][118][119] and to induce chromosomal rearrangements.[120][121] Increased transposon activation, as proposed in McClintock's ‘genomic shock’ theory, could result in alterations to gene expression. Transposable elements may, in addition to altering gene products if inserted into a gene, also alter promoter activity for genes if inserted upstream of the coding regions, or may induce gene silencing as a result of gene disruption.[122][123] For allopolyploid genomes chromosomal rearrangements may result from the ”genomic shock” induced by hybridisation, with more distantly related species being more prone to genome reorganisations e.g. in Nicotiana.[124] Chromosomal rearrangements resulting from either genomic shock or recombination events between non-homologous subgenomes may cause genome sizes to either increase or decrease.[125] Both increases and decreases were found in the Nicotiana genus, and were not related to the age since hybridization.[126]
Following genome duplication in allopolyploids, the genome goes through diploidization, which is a process in which the genome is rearranged to act as a meiotic diploid. [127][128] After such diploidization, much of the genome is lost due to genome fractionation, the loss-of-function of one or the other of the newly duplicated genes.[128][129] In a meta analysis, Sankoff and collaborators found evidence consistent with reduction-resistant pairs and a concentration of functional genes on a single chromosome and suggest that the reduction process partly is constrained.[129]
A related allopolyploid specific phenomenon is subgenome dominance. For example, in the octoploid Fragaria strawberry, one of the four subgenomes is dominant and has significantly greater gene content, more frequently has its genes expressed, and exchanges between homologous chromosomes are biased in favour of this subgenome, as compared with the other subgenomes.[130] This study also showed that certain traits, e.g. disease-resistance, are controlled by the dominant subgenome to a high extent.[130] A proposed mechanism of how subgenome dominance arises, suggests that relative dominance is related to the density of transposable elements in each subgenome. Subgenomes with higher transposable element density tend to behave submissively relative to the other subgenomes when brought together in the allopolyploid genome.[128][131] Interestingly, subgenome dominance can arise immediately in allopolyploids, as shown in synthetic and recently evolved monkeyflowers.[131]
In addition to these changes to genome structure and properties, studies of allopolyploid rice and whitefish suggest that patterns of gene expression may be disrupted in hybrid species.[132][133] Studies of synthetic and natural allopolyploids of Tragopogon miscellus show that gene expression is less strictly regulated directly after hybridization, and that novel patterns of expression emerge and are stabilized during 40 generations.[134] While expression variation in miRNAs alters gene expression and affects growth in the natural allopolyploid Arabidopsis suecica and experimental lineages, inheritance of siRNAs is stable and maintains chromatin and genome stability,[135] potentially buffering against a transcriptomic shock.
Factors influencing formation and persistence
Whereas hybridization is required for the generation of persistent hybrid genomes, it is not sufficient. For the persistence of hybrid genomes in hybrid species they need to be sufficiently reproductively isolated from their parent species to avoid species fusion. Selection on introgressed variants allows the persistence of hybrid genomes in introgressed lineages. Frequency of hybridization, viability of hybrids, and the ease at which reproductive isolation against the parent species arises or strength of selection to maintain introgressed regions are hence factors influencing the rate of formation of stable hybrid lineages.
Few general conclusions about the relative prevalence of hybridization can be drawn, as sampling is not evenly distributed, even if there is evidence for hybridization in an increasing number of taxa. One pattern that emerges is that hybridization is more frequent in plants where it occurs in 25% of the species, whereas it only occurs in 10% of animal species.[136] Most plants, as well as many groups of animals, lack heteromorphic sex chromosomes.[137] The absence of heteromorphic sex chromosomes results in slower accumulation of reproductive isolation,[138][139] and may hence enable hybridization between phylogenetically more distant taxa. Haldane's rule[140] states that ”when F1 offspring of two different animal races one sex is absent, rare, or sterile, that sex is the heterozygous sex”. Empirical evidence supports a role for heteromorphic sex chromosomes in hybrid sterility and inviability. A closely related observation is the large X effect stating that there is a disproportionate contribution of the X/Z-chromosome in fitness reduction of heterogametic hybrids.[22] These patterns likely arise as recessive alleles with deleterious effects in hybrids have a stronger impacts on the heterogametic than the homogametic sex, due to hemizygous expression.[141] In taxa with well-differentiated sex chromosomes, Haldane’s rule has shown to be close to universal, and heteromorphic sex chromosomes show reduced introgression on the X in XY.[142] In line with a role for heteromorphic sex chromosomes in constraining hybrid genome formation, elevated differentiation on sex chromosomes has been observed in both ZW and XY systems.[143] This pattern may reflect the lower effective population sizes and higher susceptibility to drift on the sex chromosomes,[144] the elevated frequency of loci involved in reproductive isolation[145] and/or the heightened conflict on sex chromosomes.[146] Findings of selection for uniparental inheritance of e.g. mitonuclear loci residing on the Z chromosome in hybrid Italian sparrows[75] is consistent with compatible sex chromosomes being important for the formation of a viable hybrid genomes.
There are also several ecological factors that affect the probability of hybridization. Generally, hybridization is more frequently observed in species with external fertilization including plants but also fishes, than in internally fertilized clades.[4] In plants, high rates of selfing in some species may prevent hybridization, and breeding system may also affect the frequency of heterospecific pollen transfer.[147][148] In fungi, hybrids can be generated by ameiotic fusion of cells or hyphae[149] in addition to mechanisms available to plants and animals. Such fusion of vegetative cells and subsequent parasexual mating with mitotic crossover may generate recombined hybrid cells.[149]
For hybrid species to evolve, reproductive isolation against the parent species is required. The ease by which such reproductive isolation arises is thus also important for the rate at which stable hybrid species arise. Polyploidisation and asexuality are both mechanisms that result in instantaneous isolation and may increase the rate of hybrid lineage formation. The ability to self-pollinate may also act in favour of stabilising allopolyploid taxa by providing a compatible mate (itself) in the early stages of allopolyploid speciation when rare cytotypes are at a reproductive disadvantage due to inter-cytotype mating.[150] Selfing is also expected to increase the likelihood of establishment for homoploid hybrids according to a modelling study,[151] and the higher probability of selfing may contribute to the higher frequency of hybrid species in plants. Fungal hybridization may result in asexual hybrid species, as Epichloe fungi where hybrids species are asexual while nonhybrids include both asexual and sexual species.[152] Hybridization between strongly divergent animal taxa may also generate asexual hybrid species, as shown e.g. in the European spined loaches, Cobitis,[153] and most if not all asexual vertebrate species are of hybrid origin.[154] Interestingly, Arctic floras harbour an unusually high proportion of allopolyploid plants,[155] suggesting that these hybrid taxa could have an advantage in extreme environments, potentially through reducing the negative effects of inbreeding. Hence both genomic architecture and ecological properties may affect the probability of hybrid species formation.
For introgressed taxa, the strength of selection on introgressed variants decides whether introgressed sections will spread in the population and stable introgressed genomes will be formed. Strong selection for insecticide resistance has been shown to increase introgression of an Anopheles gambiae resistance allele into A. coluzzi malaria mosquitoes.[156] In Heliconius butterflies, strong selection on having the locally abundant wing colour patterns repeatedly led to fixation of alleles that introgressed from locally adapted butterflies into newly colonizing species or subspecies.[34] Chances of fixation of beneficial introgressed variants depend on the type and strength of selection on the introgressed variant and linkage with other introgressed variants that are selected against.
Factors influencing affected genes and genomic regions
Genetic exchange can occur between populations or incipient species diverging in geographical proximity or between divergent taxa that come into secondary contact. Hybridization between more diverged lineages is expected to have a greater potential to contribute beneficial alleles or generate novelty than hybridization between less diverged populations because more divergent alleles are combined, and are thus more likely to have a large fitness effect, to generate transgressive phenotypes.[157] Hybridization between more diverged lineages is also more likely to generate incompatible allele combinations, reducing initial hybrid fitness[158] but potentially also contributing to hybrid speciation if they are sorted reciprocally as described above.[157] An intermediate genetic distance may thus be most conducive to hybrid speciation.[157] Experimental lab crosses support this hypothesis.[91]
The proportion of the genome that is inherited from the recipient of introgressed material varies strongly among and within species. After the initial hybridization event the representation is 50% in many polyploid taxa, although parental gene copies are successively lost and might bias the contribution to one majority parent genome.[159] Relatively equal parental contributions are also found in some homoploid hybrid species[74] but in other cases they are highly unequal such as in some Heliconius species.[160] The majority ancestry may even be that from the donor of introgressed material, as was shown for Anopheles gambiae mosquitoes.[161] Interestingly there may also be variation in parental contribution within a hybrid species. In both swordtail fish and Italian sparrows there are populations which differ strongly in what proportions of the parent genomes they have inherited.[69][70]
Patterns of introgression can vary strongly across the genome, even over short chromosomal distances. Examples of adaptive introgression of well defined regions, include an inversed region containing genes involved in insecticide resistance[21] and introgression of a divergent, inverted chromosomal segment has resulted in a ”super gene” that encodes mimicry polymorphism in the butterfly Heliconius numata.[162] These findings are consistent with models suggesting that genomic rearrangements are important for the coupling of locally adaptive loci.[163] Genes and genomic regions that are adaptive may be readily introgressed between species e.g. in hybrid zones if they are not linked to incompatibility loci. This often referred to semi-permeable species boundaries,[19][164][165] and examples include e.g. genes involved in olfaction that are introgressed across a Mus musculus and M. domesticus hybrid zone.[166] In hybrid zones with mainly permeable species boundaries, patterns of introgressed regions enable deducing what genomic regions involved in incompatibilities and reproductive isolation.[167]
References
![]() This article was adapted from the following source under a CC BY 4.0 license (2019) (reviewer reports): "Eukaryote hybrid genomes". PLOS Genetics. 15 (11): e1008404. 27 November 2019. doi:10.1371/JOURNAL.PGEN.1008404. ISSN 1553-7390. PMC 6880984. PMID 31774811. Wikidata Q86320147.
This article was adapted from the following source under a CC BY 4.0 license (2019) (reviewer reports): "Eukaryote hybrid genomes". PLOS Genetics. 15 (11): e1008404. 27 November 2019. doi:10.1371/JOURNAL.PGEN.1008404. ISSN 1553-7390. PMC 6880984. PMID 31774811. Wikidata Q86320147.
- Abbott R, Albach D, Ansell S, Arntzen JW, Baird SJ, Bierne N, et al. (February 2013). "Hybridization and speciation". Journal of Evolutionary Biology. 26 (2): 229–46. doi:10.1111/j.1420-9101.2012.02599.x. PMID 23323997. S2CID 830823.
- Fisher RA (1930). The genetical theory of natural selection. Oxford: Clarendon Press. doi:10.5962/bhl.title.27468.
- Mayr E (1963). Animal Species and Evolution. Cambridge, MA and London, England: Harvard University Press. doi:10.4159/harvard.9780674865327. ISBN 9780674865327.
- Stebbins GL (1959). "The Role of Hybridization in Evolution". Proceedings of the American Philosophical Society. 103 (2): 231–251. ISSN 0003-049X. JSTOR 985151.
- Anderson E, Stebbins GL (1954). "Hybridization as an evolutionary stimulus". Evolution. 8 (4): 378–388. doi:10.1111/j.1558-5646.1954.tb01504.x.
- Arnold ML (1997). Natural Hybridization and Evolution. Cary: Oxford University Press. ISBN 9780195356687. OCLC 960164734.
- Mallet J, Besansky N, Hahn MW (February 2016). "How reticulated are species?". BioEssays. 38 (2): 140–9. doi:10.1002/bies.201500149. PMC 4813508. PMID 26709836.
- Lamichhaney S, Han F, Webster MT, Andersson L, Grant BR, Grant PR (January 2018). "Rapid hybrid speciation in Darwin's finches". Science. 359 (6372): 224–228. Bibcode:2018Sci...359..224L. doi:10.1126/science.aao4593. PMID 29170277.
- Meier JI, Marques DA, Mwaiko S, Wagner CE, Excoffier L, Seehausen O (February 2017). "Ancient hybridization fuels rapid cichlid fish adaptive radiations". Nature Communications. 8 (1): 14363. Bibcode:2017NatCo...814363M. doi:10.1038/ncomms14363. PMC 5309898. PMID 28186104.
- Mavárez J, Salazar CA, Bermingham E, Salcedo C, Jiggins CD, Linares M (June 2006). "Speciation by hybridization in Heliconius butterflies". Nature. 441 (7095): 868–71. Bibcode:2006Natur.441..868M. doi:10.1038/nature04738. PMID 16778888.
- Salazar C, Baxter SW, Pardo-Diaz C, Wu G, Surridge A, Linares M, et al. (April 2010). Walsh B (ed.). "Genetic evidence for hybrid trait speciation in heliconius butterflies". PLOS Genetics. 6 (4): e1000930. doi:10.1371/journal.pgen.1000930. PMC 2861694. PMID 20442862.
- Melo MC, Salazar C, Jiggins CD, Linares M (June 2009). "Assortative mating preferences among hybrids offers a route to hybrid speciation". Evolution; International Journal of Organic Evolution. 63 (6): 1660–5. doi:10.1111/j.1558-5646.2009.00633.x. PMID 19492995.
- Carlquist SJ, Baldwin BG, Carr GD (2003). Tarweeds & silverswords : evolution of the Madiinae (Asteraceae). St. Louis: Missouri Botanical Garden Press. ISBN 1930723202. OCLC 52892451.
- Wolf DE, Takebayashi N, Rieseberg LH (2001). "Predicting the Risk of Extinction through Hybridization". Conservation Biology. 15 (4): 1039–1053. doi:10.1046/j.1523-1739.2001.0150041039.x. ISSN 0888-8892.
- Prentis PJ, White EM, Radford IJ, Lowe AJ, Clarke AR (2007). "Can hybridization cause local extinction: a case for demographic swamping of the Australian native Senecio pinnatifolius by the invasive Senecio madagascariensis?" (PDF). The New Phytologist. 176 (4): 902–12. doi:10.1111/j.1469-8137.2007.02217.x. PMID 17850249.
- Servedio MR, Noor MA (2003). "The Role of Reinforcement in Speciation: Theory and Data". Annual Review of Ecology, Evolution, and Systematics. 34 (1): 339–364. doi:10.1146/annurev.ecolsys.34.011802.132412. ISSN 1543-592X.
- Rhymer JM, Simberloff D (1996). "Extinction by hybridization and introgression". Annual Review of Ecology and Systematics. 27 (1): 83–109. doi:10.1146/annurev.ecolsys.27.1.83. ISSN 0066-4162.
- Seehausen O (May 2006). "Conservation: losing biodiversity by reverse speciation". Current Biology. 16 (9): R334-7. doi:10.1016/j.cub.2006.03.080. PMID 16682344.
- Thompson JD (1994). "Harrison, R. G. (ed.). Hybrid Zones and the Evolutionary Process. Oxford University Press, Oxford. 364 pp. Price f45.00". Journal of Evolutionary Biology. 7 (5): 631–634. doi:10.1046/j.1420-9101.1994.7050631.x. ISBN 0-19-506917-X. ISSN 1010-061X.
- Dasmahapatra KK, Walters JR, Briscoe AD, Davey JW, Whibley A, Nadeau NJ, et al. (Heliconius Genome Consortium) (July 2012). "Butterfly genome reveals promiscuous exchange of mimicry adaptations among species". Nature. 487 (7405): 94–8. Bibcode:2012Natur.487...94T. doi:10.1038/nature11041. PMC 3398145. PMID 22722851.
- Hanemaaijer MJ, Collier TC, Chang A, Shott CC, Houston PD, Schmidt H, et al. (December 2018). "The fate of genes that cross species boundaries after a major hybridization event in a natural mosquito population". Molecular Ecology. 27 (24): 4978–4990. doi:10.1111/mec.14947. PMID 30447117.
- Coyne JA, Orr HA (2004). Speciation. Sunderland: Sinauer Associates. ISBN 0878930914. OCLC 55078441.
- Price TD, Bouvier MM (2002). "The evolution of F1 postzygotic incompatibilities in birds". Evolution. 56 (10): 2083–9. doi:10.1554/0014-3820(2002)056[2083:teofpi]2.0.co;2. ISSN 0014-3820. PMID 12449494.
- Stelkens RB, Young KA, Seehausen O (March 2010). "The accumulation of reproductive incompatibilities in African cichlid fish". Evolution; International Journal of Organic Evolution. 64 (3): 617–33. doi:10.1111/j.1558-5646.2009.00849.x. PMID 19796149. S2CID 10319450.
- Rebernig CA, Lafon-Placette C, Hatorangan MR, Slotte T, Köhler C (June 2015). Bomblies K (ed.). "Non-reciprocal Interspecies Hybridization Barriers in the Capsella Genus Are Established in the Endosperm". PLOS Genetics. 11 (6): e1005295. doi:10.1371/journal.pgen.1005295. PMC 4472357. PMID 26086217.
- Pritchard VL, Knutson VL, Lee M, Zieba J, Edmands S (February 2013). "Fitness and morphological outcomes of many generations of hybridization in the copepod Tigriopus californicus". Journal of Evolutionary Biology. 26 (2): 416–33. doi:10.1111/jeb.12060. PMID 23278939. S2CID 10092426.
- Rieseberg LH, Archer MA, Wayne RK (October 1999). "Transgressive segregation, adaptation and speciation". Heredity. 83 ( Pt 4) (4): 363–72. doi:10.1038/sj.hdy.6886170. PMID 10583537.
- Burke JM, Arnold ML (2001). "Genetics and the fitness of hybrids". Annual Review of Genetics. 35 (1): 31–52. doi:10.1146/annurev.genet.35.102401.085719. PMID 11700276. S2CID 26683922.
- Mallet J (March 2007). "Hybrid speciation". Nature. 446 (7133): 279–83. Bibcode:2007Natur.446..279M. doi:10.1038/nature05706. PMID 17361174.
- Vallejo-Marín M, Hiscock SJ (September 2016). "Hybridization and hybrid speciation under global change". The New Phytologist. 211 (4): 1170–87. doi:10.1111/nph.14004. hdl:1893/23581. PMID 27214560.
- Barton N, Bengtsson BO (December 1986). "The barrier to genetic exchange between hybridising populations". Heredity. 57 ( Pt 3) (3): 357–76. doi:10.1038/hdy.1986.135. PMID 3804765.
- Demon I, Haccou P, van den Bosch F (September 2007). "Introgression of resistance genes between populations: a model study of insecticide resistance in Bemisia tabaci". Theoretical Population Biology. 72 (2): 292–304. doi:10.1016/j.tpb.2007.06.005. PMID 17658572.
- Uecker H, Setter D, Hermisson J (June 2015). "Adaptive gene introgression after secondary contact". Journal of Mathematical Biology. 70 (7): 1523–80. doi:10.1007/s00285-014-0802-y. PMC 4426140. PMID 24992884.
- Pardo-Diaz C, Salazar C, Baxter SW, Merot C, Figueiredo-Ready W, Joron M, et al. (2012). R Kronforst M (ed.). "Adaptive introgression across species boundaries in Heliconius butterflies". PLOS Genetics. 8 (6): e1002752. doi:10.1371/journal.pgen.1002752. PMC 3380824. PMID 22737081.
- Arnold BJ, Lahner B, DaCosta JM, Weisman CM, Hollister JD, Salt DE, et al. (July 2016). "Borrowed alleles and convergence in serpentine adaptation". Proceedings of the National Academy of Sciences of the United States of America. 113 (29): 8320–5. doi:10.1073/pnas.1600405113. PMC 4961121. PMID 27357660.
- Racimo F, Sankararaman S, Nielsen R, Huerta-Sánchez E (June 2015). "Evidence for archaic adaptive introgression in humans". Nature Reviews. Genetics. 16 (6): 359–71. doi:10.1038/nrg3936. PMC 4478293. PMID 25963373.
- Kronforst MR, Papa R (May 2015). "The functional basis of wing patterning in Heliconius butterflies: the molecules behind mimicry". Genetics. 200 (1): 1–19. doi:10.1534/genetics.114.172387. PMC 4423356. PMID 25953905.
- Mérot C, Salazar C, Merrill RM, Jiggins CD, Joron M (June 2017). "Heliconius butterflies". Proceedings. Biological Sciences. 284 (1856): 20170335. doi:10.1098/rspb.2017.0335. PMC 5474069. PMID 28592669.
- Pritchard JK, Stephens M, Donnelly P (June 2000). "Inference of population structure using multilocus genotype data". Genetics. 155 (2): 945–59. PMC 1461096. PMID 10835412.
- Alexander DH, Novembre J, Lange K (September 2009). "Fast model-based estimation of ancestry in unrelated individuals". Genome Research. 19 (9): 1655–64. doi:10.1101/gr.094052.109. PMC 2752134. PMID 19648217.
- Lawson DJ, Hellenthal G, Myers S, Falush D (January 2012). Copenhaver GP (ed.). "Inference of population structure using dense haplotype data". PLOS Genetics. 8 (1): e1002453. doi:10.1371/journal.pgen.1002453. PMC 3266881. PMID 22291602.
- Lawson DJ, van Dorp L, Falush D (August 2018). "A tutorial on how not to over-interpret STRUCTURE and ADMIXTURE bar plots". Nature Communications. 9 (1): 3258. Bibcode:2018NatCo...9.3258L. doi:10.1038/s41467-018-05257-7. PMC 6092366. PMID 30108219.
- Kulathinal RJ, Stevison LS, Noor MA (July 2009). Nachman MW (ed.). "The genomics of speciation in Drosophila: diversity, divergence, and introgression estimated using low-coverage genome sequencing". PLOS Genetics. 5 (7): e1000550. doi:10.1371/journal.pgen.1000550. PMC 2696600. PMID 19578407.
- Green RE, Krause J, Briggs AW, Maricic T, Stenzel U, Kircher M, et al. (May 2010). "A draft sequence of the Neandertal genome". Science. 328 (5979): 710–722. Bibcode:2010Sci...328..710G. doi:10.1126/science.1188021. PMC 5100745. PMID 20448178.
- Durand EY, Patterson N, Reich D, Slatkin M (August 2011). "Testing for ancient admixture between closely related populations". Molecular Biology and Evolution. 28 (8): 2239–52. doi:10.1093/molbev/msr048. PMC 3144383. PMID 21325092.
- Peter BM (April 2016). "Admixture, Population Structure, and F-Statistics". Genetics. 202 (4): 1485–501. doi:10.1534/genetics.115.183913. PMC 4905545. PMID 26857625.
- Reich D, Thangaraj K, Patterson N, Price AL, Singh L (September 2009). "Reconstructing Indian population history". Nature. 461 (7263): 489–94. Bibcode:2009Natur.461..489R. doi:10.1038/nature08365. PMC 2842210. PMID 19779445.
- Martin SH, Davey JW, Jiggins CD (January 2015). "Evaluating the use of ABBA-BABA statistics to locate introgressed loci". Molecular Biology and Evolution. 32 (1): 244–57. doi:10.1093/molbev/msu269. PMC 4271521. PMID 25246699.
- Pease JB, Hahn MW (July 2015). "Detection and Polarization of Introgression in a Five-Taxon Phylogeny". Systematic Biology. 64 (4): 651–62. doi:10.1093/sysbio/syv023. PMID 25888025.
- Eaton DA, Ree RH (September 2013). "Inferring phylogeny and introgression using RADseq data: an example from flowering plants (Pedicularis: Orobanchaceae)". Systematic Biology. 62 (5): 689–706. doi:10.1093/sysbio/syt032. PMC 3739883. PMID 23652346.
- Pickrell JK, Pritchard JK (2012). Tang H (ed.). "Inference of population splits and mixtures from genome-wide allele frequency data". PLOS Genetics. 8 (11): e1002967. arXiv:1206.2332. Bibcode:2012arXiv1206.2332P. doi:10.1371/journal.pgen.1002967. PMC 3499260. PMID 23166502.
- Patterson N, Moorjani P, Luo Y, Mallick S, Rohland N, Zhan Y, et al. (November 2012). "Ancient admixture in human history". Genetics. 192 (3): 1065–93. doi:10.1534/genetics.112.145037. PMC 3522152. PMID 22960212.
- Lipson M, Loh PR, Levin A, Reich D, Patterson N, Berger B (August 2013). "Efficient moment-based inference of admixture parameters and sources of gene flow". Molecular Biology and Evolution. 30 (8): 1788–802. doi:10.1093/molbev/mst099. PMC 3708505. PMID 23709261.
- Yu Y, Barnett RM, Nakhleh L (September 2013). "Parsimonious inference of hybridization in the presence of incomplete lineage sorting". Systematic Biology. 62 (5): 738–51. doi:10.1093/sysbio/syt037. PMC 3739885. PMID 23736104.
- Wen D, Yu Y, Nakhleh L (May 2016). Edwards S (ed.). "Bayesian Inference of Reticulate Phylogenies under the Multispecies Network Coalescent". PLOS Genetics. 12 (5): e1006006. doi:10.1371/journal.pgen.1006006. PMC 4856265. PMID 27144273.
- Moorjani P, Patterson N, Hirschhorn JN, Keinan A, Hao L, Atzmon G, et al. (April 2011). McVean G (ed.). "The history of African gene flow into Southern Europeans, Levantines, and Jews". PLOS Genetics. 7 (4): e1001373. doi:10.1371/journal.pgen.1001373. PMC 3080861. PMID 21533020.
- Moorjani P, Sankararaman S, Fu Q, Przeworski M, Patterson N, Reich D (May 2016). "A genetic method for dating ancient genomes provides a direct estimate of human generation interval in the last 45,000 years". Proceedings of the National Academy of Sciences of the United States of America. 113 (20): 5652–7. Bibcode:2016PNAS..113.5652M. doi:10.1073/pnas.1514696113. PMC 4878468. PMID 27140627.
- Loh PR, Lipson M, Patterson N, Moorjani P, Pickrell JK, Reich D, Berger B (April 2013). "Inferring admixture histories of human populations using linkage disequilibrium". Genetics. 193 (4): 1233–54. doi:10.1534/genetics.112.147330. PMC 3606100. PMID 23410830.
- Sankararaman S, Patterson N, Li H, Pääbo S, Reich D (2012). Akey JM (ed.). "The date of interbreeding between Neandertals and modern humans". PLOS Genetics. 8 (10): e1002947. arXiv:1208.2238. Bibcode:2012arXiv1208.2238S. doi:10.1371/journal.pgen.1002947. PMC 3464203. PMID 23055938.
- Pinho C, Hey J (2010). "Divergence with Gene Flow: Models and Data". Annual Review of Ecology, Evolution, and Systematics. 41 (1): 215–230. doi:10.1146/annurev-ecolsys-102209-144644. ISSN 1543-592X. S2CID 45813707.
- Excoffier L, Dupanloup I, Huerta-Sánchez E, Sousa VC, Foll M (October 2013). Akey JM (ed.). "Robust demographic inference from genomic and SNP data". PLOS Genetics. 9 (10): e1003905. doi:10.1371/journal.pgen.1003905. PMC 3812088. PMID 24204310.
- Gutenkunst RN, Hernandez RD, Williamson SH, Bustamante CD (October 2009). McVean G (ed.). "Inferring the joint demographic history of multiple populations from multidimensional SNP frequency data". PLOS Genetics. 5 (10): e1000695. arXiv:0909.0925. Bibcode:2009arXiv0909.0925G. doi:10.1371/journal.pgen.1000695. PMC 2760211. PMID 19851460.
- Beaumont MA (2010). "Approximate Bayesian Computation in Evolution and Ecology". Annual Review of Ecology, Evolution, and Systematics. 41 (1): 379–406. doi:10.1146/annurev-ecolsys-102209-144621.
- Theunert C, Slatkin M (February 2017). "Distinguishing recent admixture from ancestral population structure". Genome Biology and Evolution. 9 (3): 427–437. doi:10.1093/gbe/evx018. PMC 5381645. PMID 28186554.
- Schumer M, Rosenthal GG, Andolfatto P (June 2014). "How common is homoploid hybrid speciation?". Evolution; International Journal of Organic Evolution. 68 (6): 1553–60. doi:10.1111/evo.12399. PMID 24620775. S2CID 22702297.
- Nieto Feliner G, Álvarez I, Fuertes-Aguilar J, Heuertz M, Marques I, Moharrek F, et al. (June 2017). "Is homoploid hybrid speciation that rare? An empiricist's view". Heredity. 118 (6): 513–516. doi:10.1038/hdy.2017.7. PMC 5436029. PMID 28295029.
- Rieseberg LH, Raymond O, Rosenthal DM, Lai Z, Livingstone K, Nakazato T, et al. (August 2003). "Major ecological transitions in wild sunflowers facilitated by hybridization". Science. 301 (5637): 1211–6. Bibcode:2003Sci...301.1211R. doi:10.1126/science.1086949. PMID 12907807. S2CID 9232157.
- Grant V (1981). Plant speciation (2nd ed.). New York: Columbia University Press. ISBN 0231051123. OCLC 7552165.
- Schumer M, Xu C, Powell DL, Durvasula A, Skov L, Holland C, et al. (May 2018). "Natural selection interacts with recombination to shape the evolution of hybrid genomes". Science. 360 (6389): 656–660. Bibcode:2018Sci...360..656S. doi:10.1126/science.aar3684. PMC 6069607. PMID 29674434.
- Runemark A, Trier CN, Eroukhmanoff F, Hermansen JS, Matschiner M, Ravinet M, et al. (March 2018). "Variation and constraints in hybrid genome formation". Nature Ecology & Evolution. 2 (3): 549–556. doi:10.1038/s41559-017-0437-7. PMID 29335572.
- Buerkle CA, Rieseberg LH (February 2008). "The rate of genome stabilization in homoploid hybrid species". Evolution; International Journal of Organic Evolution. 62 (2): 266–75. doi:10.1111/j.1558-5646.2007.00267.x. PMC 2442919. PMID 18039323.
- Ungerer MC, Baird SJ, Pan J, Rieseberg LH (September 1998). "Rapid hybrid speciation in wild sunflowers". Proceedings of the National Academy of Sciences of the United States of America. 95 (20): 11757–62. Bibcode:1998PNAS...9511757U. doi:10.1073/pnas.95.20.11757. PMC 21713. PMID 9751738.
- Lai Z, Nakazato T, Salmaso M, Burke JM, Tang S, Knapp SJ, Rieseberg LH (September 2005). "Extensive chromosomal repatterning and the evolution of sterility barriers in hybrid sunflower species". Genetics. 171 (1): 291–303. doi:10.1534/genetics.105.042242. PMC 1456521. PMID 16183908.
- Elgvin TO, Trier CN, Tørresen OK, Hagen IJ, Lien S, Nederbragt AJ, et al. (June 2017). "The genomic mosaicism of hybrid speciation". Science Advances. 3 (6): e1602996. Bibcode:2017SciA....3E2996E. doi:10.1126/sciadv.1602996. PMC 5470830. PMID 28630911.
- Runemark A, Trier CN, Eroukhmanoff F, Hermansen JS, Matschiner M, Ravinet M, et al. (March 2018). "Variation and constraints in hybrid genome formation". Nature Ecology & Evolution. 2 (3): 549–556. doi:10.1038/s41559-017-0437-7. PMID 29335572.
- Otto SP, Whitton J (2000). "Polyploid incidence and evolution". Annual Review of Genetics. 34 (1): 401–437. doi:10.1146/annurev.genet.34.1.401. PMID 11092833.
- Abbott RJ, Rieseberg LH (2012). Hybrid Speciation. eLS. John Wiley & Sons, Ltd. doi:10.1002/9780470015902.a0001753.pub2. ISBN 9780470016176.
- Coyne JA (October 1989). "Mutation rates in hybrids between sibling species of Drosophila". Heredity. 63 ( Pt 2) (2): 155–62. doi:10.1038/hdy.1989.87. PMID 2553645.
- Chase MW, Paun O, Fay MF (2010). "Hybridization and speciation in angiosperms: arole for pollinator shifts?". Journal of Biology. 9 (3): 21. doi:10.1186/jbiol231. ISSN 1475-4924.
- Grant V (March 1949). "Pollination systems as isolating mechanisms in angiosperms". Evolution; International Journal of Organic Evolution. 3 (1): 82–97. doi:10.1111/j.1558-5646.1949.tb00007.x. PMID 18115119.
- Segraves KA, Thompson JN (August 1999). "Plant Polyploidy and Pollination: Floral Traits and Insect Visits to Diploid and Tetraploidheuchera Grossulariifolia". Evolution; International Journal of Organic Evolution. 53 (4): 1114–1127. doi:10.1111/j.1558-5646.1999.tb04526.x. PMID 28565509.
- Moe AM, Weiblen GD (December 2012). "Pollinator-mediated reproductive isolation among dioecious fig species (Ficus, Moraceae)". Evolution; International Journal of Organic Evolution. 66 (12): 3710–21. doi:10.1111/j.1558-5646.2012.01727.x. PMID 23206130.
- Lowe AJ, Abbott RJ (May 2004). "Reproductive isolation of a new hybrid species, Senecio eboracensis Abbott & Lowe (Asteraceae)". Heredity. 92 (5): 386–95. doi:10.1038/sj.hdy.6800432. PMID 15014422.
- Selz OM, Thommen R, Maan ME, Seehausen O (February 2014). "Behavioural isolation may facilitate homoploid hybrid speciation in cichlid fish". Journal of Evolutionary Biology. 27 (2): 275–89. doi:10.1111/jeb.12287. PMID 24372872.
- Schwarzbach AE, Donovan LA, Rieseberg LH (2001). "Transgressive character expression in a hybrid sunflower species". American Journal of Botany. 88 (2): 270–277. doi:10.2307/2657018. ISSN 0002-9122. JSTOR 2657018.
- Mameli G, López-Alvarado J, Farris E, Susanna A, Filigheddu R, Garcia-Jacas N (2014). "The role of parental and hybrid species in multiple introgression events: evidence of homoploid hybrid speciation in Centaurea (Cardueae, Asteraceae): Introgression in Centaurea". Botanical Journal of the Linnean Society. 175 (3): 453–467. doi:10.1111/boj.12177.
- Xie X, Michel AP, Schwarz D, Rull J, Velez S, Forbes AA, et al. (May 2008). "Radiation and divergence in the Rhagoletis pomonella species complex: inferences from DNA sequence data". Journal of Evolutionary Biology. 21 (3): 900–13. doi:10.1111/j.1420-9101.2008.01507.x. PMID 18312319.
- Feder JL, Xie X, Rull J, Velez S, Forbes A, Leung B, et al. (May 2005). "Mayr, Dobzhansky, and Bush and the complexities of sympatric speciation in Rhagoletis". Proceedings of the National Academy of Sciences of the United States of America. 102 Suppl 1 (Supplement 1): 6573–80. Bibcode:2005PNAS..102.6573F. doi:10.1073/pnas.0502099102. PMC 1131876. PMID 15851672.
- Schumer M, Powell DL, Delclós PJ, Squire M, Cui R, Andolfatto P, Rosenthal GG (October 2017). "Assortative mating and persistent reproductive isolation in hybrids". Proceedings of the National Academy of Sciences of the United States of America. 114 (41): 10936–10941. doi:10.1073/pnas.1711238114. PMC 5642718. PMID 28973863.
- Rieseberg LH, Linder CR, Seiler GJ (November 1995). "Chromosomal and genic barriers to introgression in Helianthus". Genetics. 141 (3): 1163–71. PMC 1206838. PMID 8582621.
- Comeault AA, Matute DR (September 2018). "Genetic divergence and the number of hybridizing species affect the path to homoploid hybrid speciation". Proceedings of the National Academy of Sciences of the United States of America. 115 (39): 9761–9766. doi:10.1073/pnas.1809685115. PMC 6166845. PMID 30209213.
- Blanckaert A, Bank C (September 2018). Zhang J (ed.). "In search of the Goldilocks zone for hybrid speciation". PLOS Genetics. 14 (9): e1007613. doi:10.1371/journal.pgen.1007613. PMC 6145587. PMID 30192761.
- Schumer M, Cui R, Rosenthal GG, Andolfatto P (March 2015). Payseur BA (ed.). "Reproductive isolation of hybrid populations driven by genetic incompatibilities". PLOS Genetics. 11 (3): e1005041. doi:10.1371/journal.pgen.1005041. PMC 4359097. PMID 25768654.
- Vereecken NJ, Cozzolino S, Schiestl FP (April 2010). "Hybrid floral scent novelty drives pollinator shift in sexually deceptive orchids". BMC Evolutionary Biology. 10 (1): 103. doi:10.1186/1471-2148-10-103. PMC 2875231. PMID 20409296.
- Gaeta RT, Chris Pires J (April 2010). "Homoeologous recombination in allopolyploids: the polyploid ratchet". The New Phytologist. 186 (1): 18–28. doi:10.1111/j.1469-8137.2009.03089.x. PMID 20002315.
- Hvala JA, Frayer ME, Payseur BA (May 2018). "Signatures of hybridization and speciation in genomic patterns of ancestry". Evolution; International Journal of Organic Evolution. 72 (8): 1540–1552. doi:10.1111/evo.13509. PMC 6261709. PMID 29806154.
- Rieseberg LH, Sinervo B, Linder CR, Ungerer MC, Arias DM (May 1996). "Role of Gene Interactions in Hybrid Speciation: Evidence from Ancient and Experimental Hybrids". Science. 272 (5262): 741–5. Bibcode:1996Sci...272..741R. doi:10.1126/science.272.5262.741. PMID 8662570. S2CID 39005242.
- Stukenbrock EH, Christiansen FB, Hansen TT, Dutheil JY, Schierup MH (July 2012). "Fusion of two divergent fungal individuals led to the recent emergence of a unique widespread pathogen species". Proceedings of the National Academy of Sciences of the United States of America. 109 (27): 10954–9. Bibcode:2012PNAS..10910954S. doi:10.1073/pnas.1201403109. PMC 3390827. PMID 22711811.
- Schumer M, Brandvain Y (June 2016). "Determining epistatic selection in admixed populations". Molecular Ecology. 25 (11): 2577–91. doi:10.1111/mec.13641. PMID 27061282.
- Sankararaman S, Mallick S, Dannemann M, Prüfer K, Kelso J, Pääbo S, et al. (March 2014). "The genomic landscape of Neanderthal ancestry in present-day humans". Nature. 507 (7492): 354–7. Bibcode:2014Natur.507..354S. doi:10.1038/nature12961. PMC 4072735. PMID 24476815.
- Eroukhmanoff F, Bailey RI, Elgvin TO, Hermansen JS, Runemark AR, Trier CN, Sætre G (2017). "Resolution of conflict between parental genomes in a hybrid species". bioRxiv. doi:10.1101/102970.
- Ohta T (1971). "Associative overdominance caused by linked detrimental mutations". Genetical Research. 18 (3): 277–286. doi:10.1017/s0016672300012684. ISSN 0016-6723.
- Zhao L, Charlesworth B (July 2016). "Resolving the Conflict Between Associative Overdominance and Background Selection". Genetics. 203 (3): 1315–34. doi:10.1534/genetics.116.188912. PMC 4937488. PMID 27182952.
- Faria R, Johannesson K, Butlin RK, Westram AM (March 2019). "Evolving Inversions". Trends in Ecology & Evolution. 34 (3): 239–248. doi:10.1016/j.tree.2018.12.005. PMID 30691998.
- Barton NH (December 2018). "The consequences of an introgression event". Molecular Ecology. 27 (24): 4973–4975. doi:10.1111/mec.14950. PMID 30599087.
- Martin SH, Davey JW, Salazar C, Jiggins CD (February 2019). Moyle L (ed.). "Recombination rate variation shapes barriers to introgression across butterfly genomes". PLOS Biology. 17 (2): e2006288. doi:10.1371/journal.pbio.2006288. PMC 6366726. PMID 30730876.
- Brandvain Y, Kenney AM, Flagel L, Coop G, Sweigart AL (June 2014). Jiggins CD (ed.). "Speciation and introgression between Mimulus nasutus and Mimulus guttatus". PLOS Genetics. 10 (6): e1004410. doi:10.1371/journal.pgen.1004410. PMC 4072524. PMID 24967630.
- Janoušek V, Munclinger P, Wang L, Teeter KC, Tucker PK (May 2015). "Functional organization of the genome may shape the species boundary in the house mouse". Molecular Biology and Evolution. 32 (5): 1208–20. doi:10.1093/molbev/msv011. PMC 4408407. PMID 25631927.
- Schumer M, Cui R, Powell DL, Dresner R, Rosenthal GG, Andolfatto P (June 2014). "High-resolution mapping reveals hundreds of genetic incompatibilities in hybridizing fish species". eLife. 3. doi:10.7554/eLife.02535. PMC 4080447. PMID 24898754.
- Liu S, Luo J, Chai J, Ren L, Zhou Y, Huang F, et al. (February 2016). "Genomic incompatibilities in the diploid and tetraploid offspring of the goldfish × common carp cross". Proceedings of the National Academy of Sciences of the United States of America. 113 (5): 1327–32. Bibcode:2016PNAS..113.1327L. doi:10.1073/pnas.1512955113. PMC 4747765. PMID 26768847.
- Trier CN, Hermansen JS, Sætre GP, Bailey RI (January 2014). Jiggins CD (ed.). "Evidence for mito-nuclear and sex-linked reproductive barriers between the hybrid Italian sparrow and its parent species". PLOS Genetics. 10 (1): e1004075. doi:10.1371/journal.pgen.1004075. PMC 3886922. PMID 24415954.
- Giordano L, Sillo F, Garbelotto M, Gonthier P (January 2018). "Mitonuclear interactions may contribute to fitness of fungal hybrids". Scientific Reports. 8 (1): 1706. Bibcode:2018NatSR...8.1706G. doi:10.1038/s41598-018-19922-w. PMC 5786003. PMID 29374209.
- Case AL, Finseth FR, Barr CM, Fishman L (September 2016). "Selfish evolution of cytonuclear hybrid incompatibility in Mimulus". Proceedings. Biological Sciences. 283 (1838): 20161493. doi:10.1098/rspb.2016.1493. PMC 5031664. PMID 27629037.
- David WM, Mitchell DL, Walter RB (July 2004). "DNA repair in hybrid fish of the genus Xiphophorus". Comparative Biochemistry and Physiology. Toxicology & Pharmacology. 138 (3): 301–9. doi:10.1016/j.cca.2004.07.006. PMID 15533788.
- Avila V, Chavarrías D, Sánchez E, Manrique A, López-Fanjul C, García-Dorado A (May 2006). "Increase of the spontaneous mutation rate in a long-term experiment with Drosophila melanogaster". Genetics. 173 (1): 267–77. doi:10.1534/genetics.106.056200. PMC 1461422. PMID 16547099.
- Bashir T, Sailer C, Gerber F, Loganathan N, Bhoopalan H, Eichenberger C, et al. (May 2014). "Hybridization alters spontaneous mutation rates in a parent-of-origin-dependent fashion in Arabidopsis". Plant Physiology. 165 (1): 424–37. doi:10.1104/pp.114.238451. PMC 4012600. PMID 24664208.
- Dennenmoser S, Sedlazeck FJ, Iwaszkiewicz E, Li XY, Altmüller J, Nolte AW (September 2017). "Copy number increases of transposable elements and protein-coding genes in an invasive fish of hybrid origin". Molecular Ecology. 26 (18): 4712–4724. doi:10.1111/mec.14134. PMC 5638112. PMID 28390096.
- Dion-Côté AM, Renaut S, Normandeau E, Bernatchez L (May 2014). "RNA-seq reveals transcriptomic shock involving transposable elements reactivation in hybrids of young lake whitefish species". Molecular Biology and Evolution. 31 (5): 1188–99. doi:10.1093/molbev/msu069. PMID 24505119.
- Senerchia N, Felber F, Parisod C (April 2015). "Genome reorganization in F1 hybrids uncovers the role of retrotransposons in reproductive isolation". Proceedings. Biological Sciences. 282 (1804): 20142874. doi:10.1098/rspb.2014.2874. PMC 4375867. PMID 25716787.
- Ostberg CO, Hauser L, Pritchard VL, Garza JC, Naish KA (August 2013). "Chromosome rearrangements, recombination suppression, and limited segregation distortion in hybrids between Yellowstone cutthroat trout (Oncorhynchus clarkii bouvieri) and rainbow trout (O. mykiss)". BMC Genomics. 14 (1): 570. doi:10.1186/1471-2164-14-570. PMC 3765842. PMID 23968234.
- Hirai H, Hirai Y, Morimoto M, Kaneko A, Kamanaka Y, Koga A (April 2017). "Night Monkey Hybrids Exhibit De Novo Genomic and Karyotypic Alterations: The First Such Case in Primates". Genome Biology and Evolution. 9 (4): 945–955. doi:10.1093/gbe/evx058. PMC 5388293. PMID 28369492.
- Barkan A, Martienssen RA (April 1991). "Inactivation of maize transposon Mu suppresses a mutant phenotype by activating an outward-reading promoter near the end of Mu1". Proceedings of the National Academy of Sciences of the United States of America. 88 (8): 3502–6. Bibcode:1991PNAS...88.3502B. doi:10.1073/pnas.88.8.3502. PMC 51476. PMID 1849660.
- Raizada MN, Benito M, Walbot V (2008). "The MuDR transposon terminal inverted repeat contains a complex plant promoter directing distinct somatic and germinal programs: Transposon promoter expression pattern". The Plant Journal. 25 (1): 79–91. doi:10.1111/j.1365-313X.2001.00939.x. S2CID 26084899.
- Lim KY, Matyasek R, Kovarik A, Leitch AR (2004). "Genome evolution in allotetraploid Nicotiana". Biological Journal of the Linnean Society. 82 (4): 599–606. doi:10.1111/j.1095-8312.2004.00344.x.
- Baack EJ, Whitney KD, Rieseberg LH (August 2005). "Hybridization and genome size evolution: timing and magnitude of nuclear DNA content increases in Helianthus homoploid hybrid species". The New Phytologist. 167 (2): 623–30. doi:10.1111/j.1469-8137.2005.01433.x. PMC 2442926. PMID 15998412.
- Leitch IJ, Hanson L, Lim KY, Kovarik A, Chase MW, Clarkson JJ, Leitch AR (April 2008). "The ups and downs of genome size evolution in polyploid species of Nicotiana (Solanaceae)". Annals of Botany. 101 (6): 805–14. doi:10.1093/aob/mcm326. PMC 2710205. PMID 18222910.
- Wolfe KH (May 2001). "Yesterday's polyploids and the mystery of diploidization". Nature Reviews. Genetics. 2 (5): 333–41. doi:10.1038/35072009. PMID 11331899.
- Freeling M, Scanlon MJ, Fowler JE (December 2015). "Fractionation and subfunctionalization following genome duplications: mechanisms that drive gene content and their consequences". Current Opinion in Genetics & Development. 35: 110–8. doi:10.1016/j.gde.2015.11.002. PMID 26657818.
- Sankoff D, Zheng C, Zhu Q (May 2010). "The collapse of gene complement following whole genome duplication". BMC Genomics. 11 (1): 313. doi:10.1186/1471-2164-11-313. PMC 2896955. PMID 20482863.
- Edger PP, Poorten TJ, VanBuren R, Hardigan MA, Colle M, McKain MR, et al. (March 2019). "Origin and evolution of the octoploid strawberry genome". Nature Genetics. 51 (3): 541–547. doi:10.1038/s41588-019-0356-4. PMC 6882729. PMID 30804557.
- Edger PP, Smith R, McKain MR, Cooley AM, Vallejo-Marin M, Yuan Y, et al. (September 2017). "Subgenome Dominance in an Interspecific Hybrid, Synthetic Allopolyploid, and a 140-Year-Old Naturally Established Neo-Allopolyploid Monkeyflower". The Plant Cell. 29 (9): 2150–2167. doi:10.1105/tpc.17.00010. PMC 5635986. PMID 28814644.
- Xu C, Bai Y, Lin X, Zhao N, Hu L, Gong Z, et al. (May 2014). "Genome-wide disruption of gene expression in allopolyploids but not hybrids of rice subspecies". Molecular Biology and Evolution. 31 (5): 1066–76. doi:10.1093/molbev/msu085. PMC 3995341. PMID 24577842.
- Renaut S, Nolte AW, Bernatchez L (April 2009). "Gene expression divergence and hybrid misexpression between lake whitefish species pairs (Coregonus spp. Salmonidae)". Molecular Biology and Evolution. 26 (4): 925–36. doi:10.1093/molbev/msp017. PMID 19174479.
- Buggs RJ, Zhang L, Miles N, Tate JA, Gao L, Wei W, et al. (April 2011). "Transcriptomic shock generates evolutionary novelty in a newly formed, natural allopolyploid plant". Current Biology. 21 (7): 551–6. doi:10.1016/j.cub.2011.02.016. PMID 21419627.
- Ha M, Lu J, Tian L, Ramachandran V, Kasschau KD, Chapman EJ, et al. (October 2009). "Small RNAs serve as a genetic buffer against genomic shock in Arabidopsis interspecific hybrids and allopolyploids". Proceedings of the National Academy of Sciences of the United States of America. 106 (42): 17835–40. Bibcode:2009PNAS..10617835H. doi:10.1073/pnas.0907003106. PMC 2757398. PMID 19805056.
- Mallet J (May 2005). "Hybridization as an invasion of the genome". Trends in Ecology & Evolution. 20 (5): 229–37. doi:10.1016/j.tree.2005.02.010. PMID 16701374.
- Charlesworth D (April 2016). "Plant Sex Chromosomes". Annual Review of Plant Biology. 67 (1): 397–420. doi:10.1146/annurev-arplant-043015-111911. PMID 26653795.
- Rieseberg LH (2001). "Chromosomal rearrangements and speciation". Trends in Ecology & Evolution. 16 (7): 351–358. doi:10.1016/s0169-5347(01)02187-5. ISSN 0169-5347. PMID 11403867.
- Levin DA (November 2012). "The long wait for hybrid sterility in flowering plants". The New Phytologist. 196 (3): 666–70. doi:10.1111/j.1469-8137.2012.04309.x. PMID 22966819.
- Haldane JB (1922). "Sex ratio and unisexual sterility in hybrid animals". Journal of Genetics. 12 (2): 101–109. doi:10.1007/BF02983075. ISSN 0022-1333.
- Turelli M, Orr HA (May 1995). "The dominance theory of Haldane's rule". Genetics. 140 (1): 389–402. PMC 1206564. PMID 7635302.
- Runemark A, Eroukhmanoff F, Nava-Bolaños A, Hermansen JS, Meier JI (October 2018). "Hybridization, sex-specific genomic architecture and local adaptation". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 373 (1757): 20170419. doi:10.1098/rstb.2017.0419. PMC 6125728. PMID 30150218.
- Payseur BA, Rieseberg LH (June 2016). "A genomic perspective on hybridization and speciation". Molecular Ecology. 25 (11): 2337–60. doi:10.1111/mec.13557. PMC 4915564. PMID 26836441.
- Lynch M (1998). Genetics and analysis of quantitative traits. Walsh, Bruce, 1957-. Sunderland, Mass.: Sinauer. ISBN 0878934812. OCLC 37030646.
- Masly JP, Presgraves DC (September 2007). Barton NH (ed.). "High-resolution genome-wide dissection of the two rules of speciation in Drosophila". PLOS Biology. 5 (9): e243. doi:10.1371/journal.pbio.0050243. PMC 1971125. PMID 17850182.
- Mank JE, Hosken DJ, Wedell N (October 2014). "Conflict on the sex chromosomes: cause, effect, and complexity". Cold Spring Harbor Perspectives in Biology. 6 (12): a017715. doi:10.1101/cshperspect.a017715. PMC 4292157. PMID 25280765.
- Brys R, Vanden Broeck A, Mergeay J, Jacquemyn H (May 2014). "The contribution of mating system variation to reproductive isolation in two closely related Centaurium species (Gentianaceae) with a generalized flower morphology". Evolution; International Journal of Organic Evolution. 68 (5): 1281–93. doi:10.1111/evo.12345. PMID 24372301.
- Widmer A, Lexer C, Cozzolino S (January 2009). "Evolution of reproductive isolation in plants". Heredity. 102 (1): 31–8. doi:10.1038/hdy.2008.69. PMID 18648386.
- Schardl CL, Craven KD (November 2003). "Interspecific hybridization in plant-associated fungi and oomycetes: a review". Molecular Ecology. 12 (11): 2861–73. doi:10.1046/j.1365-294x.2003.01965.x. PMID 14629368.
- Levin DA (1975). "Minority Cytotype Exclusion in Local Plant Populations". Taxon. 24 (1): 35–43. doi:10.2307/1218997. JSTOR 1218997.
- McCarthy EM, Asmussen MA, Anderson WW (1995). "A theoretical assessment of recombinational speciation". Heredity. 74 (5): 502–509. doi:10.1038/hdy.1995.71. ISSN 0018-067X.
- Charlton ND, Craven KD, Afkhami ME, Hall BA, Ghimire SR, Young CA (October 2014). "Interspecific hybridization and bioactive alkaloid variation increases diversity in endophytic Epichloë species of Bromus laevipes". FEMS Microbiology Ecology. 90 (1): 276–89. doi:10.1111/1574-6941.12393. PMID 25065688.
- Janko K, Pačes J, Wilkinson-Herbots H, Costa RJ, Roslein J, Drozd P, et al. (January 2018). "Hybrid asexuality as a primary postzygotic barrier between nascent species: On the interconnection between asexuality, hybridization and speciation". Molecular Ecology. 27 (1): 248–263. doi:10.1111/mec.14377. PMC 6849617. PMID 28987005.
- Neaves WB, Baumann P (March 2011). "Unisexual reproduction among vertebrates". Trends in Genetics. 27 (3): 81–8. doi:10.1016/j.tig.2010.12.002. PMID 21334090.
- Brochmann C, Brysting AK, Alsos IG, Borgen L, Grundt HH, Scheen AC, Elven R (2004). "Polyploidy in arctic plants". Biological Journal of the Linnean Society. 82 (4): 521–536. doi:10.1111/j.1095-8312.2004.00337.x.
- Norris LC, Main BJ, Lee Y, Collier TC, Fofana A, Cornel AJ, Lanzaro GC (January 2015). "Adaptive introgression in an African malaria mosquito coincident with the increased usage of insecticide-treated bed nets". Proceedings of the National Academy of Sciences of the United States of America. 112 (3): 815–20. Bibcode:2015PNAS..112..815N. doi:10.1073/pnas.1418892112. PMC 4311837. PMID 25561525.
- Marques DA, Meier JI, Seehausen O (June 2019). "A Combinatorial View on Speciation and Adaptive Radiation". Trends in Ecology & Evolution. 34 (6): 531–544. doi:10.1016/j.tree.2019.02.008. PMID 30885412.
- Maheshwari S, Barbash DA (2011). "The genetics of hybrid incompatibilities". Annual Review of Genetics. 45 (1): 331–55. doi:10.1146/annurev-genet-110410-132514. PMID 21910629.
- Buggs RJ, Doust AN, Tate JA, Koh J, Soltis K, Feltus FA, et al. (July 2009). "Gene loss and silencing in Tragopogon miscellus (Asteraceae): comparison of natural and synthetic allotetraploids". Heredity. 103 (1): 73–81. doi:10.1038/hdy.2009.24. PMID 19277058.
- Jiggins CD, Salazar C, Linares M, Mavarez J (September 2008). "Review. Hybrid trait speciation and Heliconius butterflies". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 363 (1506): 3047–54. doi:10.1098/rstb.2008.0065. PMC 2607310. PMID 18579480.
- Fontaine MC, Pease JB, Steele A, Waterhouse RM, Neafsey DE, Sharakhov IV, et al. (January 2015). "Mosquito genomics. Extensive introgression in a malaria vector species complex revealed by phylogenomics". Science. 347 (6217): 1258524. doi:10.1126/science.1258524. PMC 4380269. PMID 25431491.
- Jay P, Whibley A, Frézal L, Rodríguez de Cara MÁ, Nowell RW, Mallet J, et al. (June 2018). "Supergene Evolution Triggered by the Introgression of a Chromosomal Inversion". Current Biology. 28 (11): 1839–1845.e3. doi:10.1016/j.cub.2018.04.072. PMID 29804810.
- Yeaman S (May 2013). "Genomic rearrangements and the evolution of clusters of locally adaptive loci". Proceedings of the National Academy of Sciences of the United States of America. 110 (19): E1743-51. Bibcode:2013PNAS..110E1743Y. doi:10.1073/pnas.1219381110. PMC 3651494. PMID 23610436.
- Wu C (2001). "The genic view of the process of speciation: Genic view of the process of speciation". Journal of Evolutionary Biology. 14 (6): 851–865. doi:10.1046/j.1420-9101.2001.00335.x. S2CID 54863357.
- Harrison RG, Larson EL (2014). "Hybridization, introgression, and the nature of species boundaries". The Journal of Heredity. 105 Suppl 1 (S1): 795–809. doi:10.1093/jhered/esu033. PMID 25149255.
- Teeter KC, Payseur BA, Harris LW, Bakewell MA, Thibodeau LM, O'Brien JE, et al. (January 2008). "Genome-wide patterns of gene flow across a house mouse hybrid zone". Genome Research. 18 (1): 67–76. doi:10.1101/gr.6757907. PMC 2134771. PMID 18025268.
- Hooper DM, Griffith SC, Price TD (March 2019). "Sex chromosome inversions enforce reproductive isolation across an avian hybrid zone". Molecular Ecology. 28 (6): 1246–1262. doi:10.1111/mec.14874. PMID 30230092.
