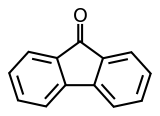
Fluorenone
Fluorenone is an aromatic organic compound with the chemical formula C13H8O. It is used to make antimalaria drugs. It can be synthesised from fluorene with the addition of glacial acetic acid and sodium hypochlorite solution, undergoing an oxidation reaction. It is bright fluorescent yellow in color and is a solid at room temperature.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Fluoren-9-one | |
| Other names
9-Fluorenone; 9H-Fluoren-9-one; 9-Oxofluorene; Diphenylene ketone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.937 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C13H8O | |
| Molar mass | 180.206 g·mol−1 |
| Appearance | Yellow solid |
| Density | 1.130 g/cm3 (99 °C)[1] |
| Melting point | 84.0 °C (183.2 °F; 357.1 K)[1] |
| Boiling point | 341.5 °C (646.7 °F; 614.6 K)[1] |
| Insoluble | |
| Solubility | soluble in alcohol, acetone, benzene very soluble in ether, toluene |
| log P | 3.58 |
| -99.4·10−6 cm3/mol | |
Refractive index (nD) |
1.6309 |
| Hazards | |
| Main hazards | Irritant |
| Safety data sheet | External MSDS |
| NFPA 704 (fire diamond) | |
| Flash point | 163 °C (325 °F; 436 K)[1] |
| 608 °C (1,126 °F; 881 K) | |
| Related compounds | |
Related compounds |
Fluorene 1,8-Diazafluoren-9-one |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
According to UBC, the derivative compound fluorenone thiosemicarbazone (CAS number 68279-50-5) can be used to counterbalance androgens.[2]
- IPS-339 is beta-blocker.
It is used as a fragrance or odor agent in candles.
Azafluorenone
Introduction
Azaflouorenones are fused tricyclic compounds that are pyridine analogs to fluorenones. In 1976, researchers discovered the first azafluorenone natural product, onychine.[3] These azafluorenones have been isolated from a variety of plants and are believed to derive from aporphine during their biosynthesis.They have shown a wide range of antimicrobial activity against several microorganisms including C. albicans, Escherichia coli and Saccharomyces cerevisiae.
Biological Importance
Azaflurenone, being the core structural unit in a wide range of natural products, has attracted much research in recent times. Representative members of this class of compounds are onychine, polyfothine, isoursuline which show powerful antimicrobial, DNA damaging, and anti-malarial effects against P.falciperum.Cyathocaline acts as a DNA modifying agent. Azafluorenone derivatives have been reported to possess activities of aldose reductase inhibition, thrombin inhibition, and is also used in organic light-emitting devices (OLED).[4]
N-oxime rearrangements
The use of N-oximes have proven versatile towards the synthesis of azafluorenones natural products.[5]
Imine condensations
In 1949, Petrow and co-workers reported the first synthesis of an azafluorenone.[6]
Diels-Alder Reaction
The Diels-Alder reaction has also been utilized to construct azafluorenones. In a synthesis of onychine, researchers made tricyclic (21) from the cycloaddition of indene with unsaturated imine.[7]
Nucleophilic Ring Closure
This is conceptually different strategy to synthesize azafluorenones involves nucleophilic attack of carbonyls to close the cyclopentanone ring. Snieckus’ group synthesized biaryl compounds via a Suzuki coupling . These intermediates were exposed to excess lithium diisopropylamide (LDA) to facilitate ring closure to make azafluorenones. The amides not only served as orthodirecting groups, but also as the carbonyl source of the azafluorenone. Snieckus’ group also reported a similar cross-coupling procedure toward the synthesis of onychine.[8]
Oxidative Intramolecular Heck Reaction
Azafluorenones by one step oxidation and cyclization of the corresponding alcohol which, in turn, can be prepared by Grignard reaction upon 2-bromopyridine-3-carboxaldehyde. Here, Grignard reagents were first prepared from suitably substituted halides in anhydrous diethyl ether. These freshly prepared Grignard reagents were then added to an anhydrous ethereal solution of 2-bromopyridine-3carboxaldehyde at 0 °C which yielded Heck precursors quantitatively. Alcohol was then subjected to cyclization under Heck reaction conditions in good yield.[9]
Intramolecular Suzuki coupling
Recently, researcher developed an approach to azafluorenones using diaryl ketones bearing a halogen at the 2 position of one of the aryl groups (prepared by deprotocupration–aroylation) in intramolecular direct palladium-catalyzed arylation reactions.[10]
Intramolecular acylation using methyl arenes as a acyl source: Synthesis of 4azafluorenones
A transition-metal-free, t-BuOOH mediated intramolecular carbonylation of arenes in 2-aryl-3-picolines via oxidative C−H functionalizations of the methyl group has been developed in our group, providing an expedient synthesis of 4-azafluorenones. methylarenes have been used as acylating agents, 2-aryl-3picolines in this study are transformed into aldehydes, which give 4-azafluorenones upon rapid intramolecular acylation.[11]
Intramolecular Acylation using Hydroxymethyl group: Synthesis of 4- azafluorenones
Hydroxymethyl group was used as acyl surrogate and the reaction followed via multiple C−H functionalizations yielded 4-azafluorenone [12]
Intramolecular Minisci acylation under silver-free neutral conditions
Recently intramolecular decarboxylative acylation has been developed in our group for the synthesis of 1- and 3-azafluorenones. They have developed a protocol for the intramolecular acylation of unactivated pyridines under silver-free neutral condition[13]
See also
References
- Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- De Almeida, M.Elita L.; Braz F, Raimundo; von Bülow, Vittoria; Gottlieb, Otto R.; Maia, J.Guilherme S. (January 1976). "Onychine, an alkaloid from Onychopetalum amazonicum". Phytochemistry. 15 (7): 1186–1187. doi:10.1016/0031-9422(76)85134-5. ISSN 0031-9422.
- Dhara, Shubhendu; Ahmed, Atiur; Nandi, Sukla; Baitalik, Shantanu; Ray, Jayanta K. (January 2013). "Synthesis of azafluorenone via oxidative intramolecular Heck cyclization". Tetrahedron Letters. 54 (1): 63–65. doi:10.1016/j.tetlet.2012.10.085.
- Koyama, Junko; OKatani, Teruyo; Tagahara, Kiyoshi; Irie, Hiroshi (1989). "Synthesis of Alkaloids, Cleistopholine, Oxylopine (Isoursuline), and Ursuline". Heterocycles. 29 (9): 1649. doi:10.3987/com-89-5048. ISSN 0385-5414.
- Tu, Shujiang; Jiang, Bo; Jia, Runhong; Zhang, Junyong; Zhang, Yan (February 2007). "An efficient and expeditious microwave-assisted synthesis of 4-azafluorenones via a multi-component reaction". Tetrahedron Letters. 48 (8): 1369–1374. doi:10.1016/j.tetlet.2006.12.102. ISSN 0040-4039.
- Hong, Bor‐Cherng; Hallur, Mahanandeesha Siddappa; Liao, Ju‐Hsiou (2006-06-01). "Hetero Diels–Alder Cycloaddition of Indene for the Formal Synthesis of Onychnine". Synthetic Communications. 36 (11): 1521–1528. doi:10.1080/00397910600588520. ISSN 0039-7911. S2CID 95521938.
- Alves, T.; de Oliveira, A.B.; Snieckus, V. (1988). "Short synthesis of azafluorenone alkaloids using transition metal-catalyzed cross coupling tactics". Tetrahedron Letters. 29 (18): 2135–2136. doi:10.1016/s0040-4039(00)86691-5. ISSN 0040-4039.
- Dhara, Shubhendu; Ahmed, Atiur; Nandi, Sukla; Baitalik, Shantanu; Ray, Jayanta K. (January 2013). "Synthesis of azafluorenone via oxidative intramolecular Heck cyclization". Tetrahedron Letters. 54 (1): 63–65. doi:10.1016/j.tetlet.2012.10.085. ISSN 0040-4039.
- Alessi, Manlio; Larkin, Andrew L.; Ogilvie, Kevin A.; Green, Laine A.; Lai, Sunny; Lopez, Simon; Snieckus, Victor (2007-07-10). "Directed ortho-Metalation—Boronation and Suzuki—Miyaura Cross-Coupling of Pyridine Derivatives: A One-Pot Protocol to Substituted Azabiaryls". ChemInform. 38 (28). doi:10.1002/chin.200728135. ISSN 0931-7597.
- Laha, Joydev K.; Jethava, Krupal P.; Patel, Sagarkumar (April 2016). "ChemInform Abstract: Scope of Successive C-H Functionalizations of the Methyl Group in 3-Picolines: Intramolecular Carbonylation of Arenes to the Metal-Free Synthesis of 4-Azafluorenones". ChemInform. 47 (17). doi:10.1002/chin.201617162. ISSN 0931-7597.
- Laha, Joydev K.; Jethava, Krupal P.; Patel, Sagarkumar; Patel, Ketul V. (2016-12-14). "Intramolecular Acylation of Unactivated Pyridines or Arenes via Multiple C–H Functionalizations: Synthesis of All Four Azafluorenones and Fluorenones". The Journal of Organic Chemistry. 82 (1): 76–85. doi:10.1021/acs.joc.6b02065. ISSN 0022-3263. PMID 27966934.
- Laha, Joydev K.; Patel, Ketul V.; Dubey, Gurudutt; Jethava, Krupal P. (2017). "Intramolecular Minisci acylation under silver-free neutral conditions for the synthesis of azafluorenones and fluorenones". Organic & Biomolecular Chemistry. 15 (10): 2199–2210. doi:10.1039/c7ob00077d. ISSN 1477-0520. PMID 28221391.
