Fluorinated gases
Fluorinated gases (F-gases) are man-made gases that can stay in the atmosphere for centuries and contribute to a global greenhouse effect. There are four types: hydrofluorocarbons (HFCs), perfluorocarbons (PFCs), sulfur hexafluoride (SF6) and nitrogen trifluoride (NF3). F-gases are a subgroup of the halogenated gases, the majority of which are halocarbons that include fluorine, but do not include chlorine, bromine, or iodine.
Types of F-gases
The most common F-gases are hydrofluorocarbons (HFCs), which contain hydrogen, fluorine, and carbon. They are used in a multitude of applications including commercial refrigeration, industrial refrigeration, air-conditioning systems, heat pump equipment, and as blowing agents for foams, fire extinguishants, aerosol propellants, and solvents. HFC-134a (1,1,1-Trifluoroethane) has grown to become the most abundant HFC in earth's atmosphere as of year 2015.[1]
Perfluorocarbons (PFCs) are the compounds consisting of fluorine and carbon. They are widely used in the electronics, cosmetics, and pharmaceutical industries, as well as in refrigeration when combined with other gases. PFCs were commonly used as fire extinguishants in the past and are still found in older fire protection systems. They are also a by-product of the aluminium smelting process. PFC-14 (Carbon tetrafluoride - CF4) has grown to become the most abundant PFC in earth's atmosphere as of year 2015.[1]
Sulphur hexafluoride (SF6) is used primarily as an arc suppression and insulation gas. It can be found in high-voltage switchgear and is used in the production of magnesium.
Nitrogen trifluoride (NF3) is used primarily as an etchant for microelectronics fabrication.
Use history
HFCs were developed in the 1990s to substitute for substances such as chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs). As these substances were found to deplete the ozone layer, the Montreal Protocol began to lay down provisions for them to be phased-out globally after the agreement was ratified in 1987.
PFCs and SF6 were already in use prior to the Montreal Protocol.
NF3 use has grown since the 1990s along with the rapid expansion of the microelectronics fabrication industry.
Environmental impact of F-gases
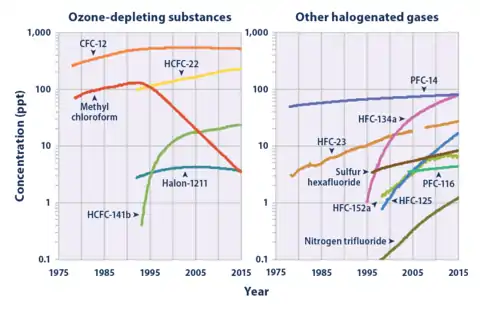
F-gases are ozone-friendly, enable energy efficiency, and are relatively safe for use by the public due to their low levels of toxicity and flammability. However, most F-gases have a high global warming potential (GWP), and some are nearly inert to removal by chemical processes. If released, HFCs stay in the atmosphere for decades and both PFCs and SF6 can stay in the atmosphere for millennia.
The total atmospheric concentration of F-gases, CFCs, and HCFCs has grown rapidly since the mid-twentieth century; a time which marks the start of their production and use at industrial scale. As a group in year 2019, these unnatural man-made gases are responsible for about one-tenth of the direct radiative forcing from all long-lived anthropogenic greenhouse gases.[2]
F-gases are used in a number of applications intended for climate change mitigation, that can generate further positive feedback for atmospheric heating. For example, refrigeration and air conditioning systems are increasingly utilized by humans within a warming environment.[3] Likewise, expansions of electrical infrastructure, as driven by the alternatives to fossil fuels, has led to rising demand for SF6.[4] If recent trends of aggressive (5% and greater CAGR) annual growth for such types of F-gas production were to continue into the future without complimentary reductions in GWP and/or atmospheric leakage, their warming influence could soon rival those of CO2 and CH4 which are trending at less than about 2% annual growth.
Regulation of F-gases
International level
Although the Montreal Protocol regulates the phasing out of HCFCs, there was no international agreement on the regulation of HFCs until late 2016 when the Kigali Agreement under the Montreal Protocol was signed, which has put compulsory phase wise phasing out of CFC gases. Efforts are ongoing to develop a global approach for the control of HFCs. Most recently, this has taken the form of a declaration of support for a global phase-down on as part of the outcomes of the "Rio+20" United Nations Conference on Sustainable Development.[5]
US-level
In the United States, the regulation of F-gases falls under the authority of the Environmental Protection Agency's overall attempts to combat greenhouse gases.[6] The United States has put forward a joint proposal with Mexico and the Federated States of Micronesia for a phase-down of HFCs by 2030.
EU-level regulation
In order to combat the potential global warming effects of F-gases, and as part of the EU's Kyoto protocol commitments, in 2006 the European Union passed two pieces of legislation controlling their use: the F-gas Regulation (EC) No 842/2006 and the Mobile Air Conditioning Directive Directive 2006/40/EC. The F-gas Regulation adopts an approach based on containment and recovery of F-gases as well as imposing obligations on reporting, training and labeling on those using F-gases.
On 26 September 2011, the Commission issued a report on the application, effects and adequacy of the Regulation, drawing from the results of an analytical study it commissioned from German environmental research institute, Öko-Recherche. A further study, conducted by the Armines Centre energétique et procédés and by Energy Research Innovation Engineering (ERIE) found that emissions reductions of up to 60% can be achieved by improving containment measures and accelerating the changeover from high GWP refrigerants to ones with lower GWP.[7]
On 7 November 2012, the European Commission published the proposal to revise the F-gas Regulation. In December 2013, the European Parliament and the Council of the EU agreed the text of the revised regulation, which shall be applied from 1 January 2015.
See also
References
- "Climate change indicators - Atmospheric concentration of greenhouse gases - Figure 4". United States Environmental Protection Agency. Retrieved 2020-09-26.
- Butler J. and Montzka S. (2020). "The NOAA Annual Greenhouse Gas Index (AGGI)". NOAA Global Monitoring Laboratory/Earth System Research Laboratories.
- "Refrigerant Market Size Worth $30.37 Billion By 2025 / CAGR: 5.3%". Grand View Research. 2018-01-31.
- Mark Williams (2020-09-13). "Sulfur Hexafluoride Market is Thriving Worldwide 2020-2027". The Daily Chronicle.
- "The future we want - Outcome document of the United Nations Conference on Sustainable Development" (PDF). United Nations. 2012-06-22. p. 39.
- "Greenhouse gas emissions". Epa.gov. Retrieved 2020-09-26.
- "Archived copy" (PDF). Archived from the original (PDF) on 2013-07-20. Retrieved 2013-01-14.CS1 maint: archived copy as title (link)