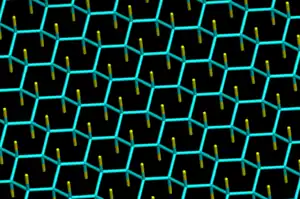
Fluorographene
Fluorographene (or perfluorographane, graphene fluoride) is a fluorocarbon derivative of graphene.[1][2][3] It is a two dimensional carbon sheet of sp3 hybridized carbons,[4] with each carbon atom bound to one fluorine. The chemical formula is (CF)
n. In comparison, Teflon (polytetrafluoroethylene), -(CF2)n-, consists of carbon "chains" with each carbon bound to two fluorines.
 | |
| Identifiers | |
|---|---|
| ChemSpider |
|
| Properties | |
| CF1(.1) | |
| Molar mass | Variable |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Unlike fluorographene, graphene is unsaturated (sp2 hybridized) and completely carbon. The hydrocarbon analogue to fluorographene is sp3 hybridized graphane. Similar to other fluorocarbons (e.g. perfluorohexane), fluorographene is highly insulating. Fluorographene is thermally stable, resembling polytetrafluoroethylene; however, chemically it is reactive. It can be transformed back into graphene by reaction with KI under higher temperature.[3] During reactions of fluorographene with NaOH and NaSH simultaneous reductive defluorination and substitution are observed. The reactivity of fluorographene represents a facile way towards graphene derivatives.[5]
Preparation
The material was first described in 2010 by Robinson et al.[1] using graphene grown on copper foil exposed to xenon difluoride at 30 °C. The group of Nair et al.[2] started from cleaved graphene crystals on a gold grid also exposed to xenon difluoride, at 70 °C. Also in 2010 Withers et al. described exfoliation of fluorinated graphite (monolayer, 24% fluorination)[6] and Cheng et al. reported reversible graphene fluorination.[7] The stoichiometric fluorographene (CF) was also prepared by chemical exfoliation of graphite fluoride by Zboril et al.[3] Zboril et al. also showed that the graphene fluoride can be transformed into graphene via graphene iodide, a spontaneously decomposing intermediate.[3]
Structure
Structure of fluorographene can be derived from the structure of graphite monofluoride (CF)
n, which consists of weakly bound stacked fluorographene layers, and its most stable conformation (predicted for the monocrystal) contains an infinite array of trans-linked cyclohexane chairs with covalent C–F bonds in an AB stacking sequence.[8] The estimated C-F distance is equal 136-138 pm, C-C 157-158 pm and C-C-C angle 110 deg.[9] Possible fluorographene conformations have been extensively investigated computationally.[10][11][12][13][14][15]
Electronic properties
Fluorographene is considered a wide gap semiconductor, because its I-V characteristics are strongly nonlinear with a nearly gate-independent resistance greater than 1 GΩ. In addition, fluorescence and NEXAFS measurements indicate band gap higher than 3.8 eV. Theoretical calculations show that estimation of fluorographene band gap is rather challenging task, as GGA functional provides band gap of 3.1 eV, hybrid (HSE06) 4.9 eV, GW 8.1 eV on top of PBE 8.1 or 8.3 eV on top of HSE06. The optical transition calculated by Bethe-Salpeter equation (BSE) is equal to 5.1 eV and points to an extremely strong exciton binding energy of 1.9 eV.[9] It has recently been demonstrated that using fluorographene as a passivation layer in Field Effect Transistors (FETs) featuring a graphene channel, carrier mobility increases significantly.[16]
Reaction
Fluorographene is susceptible for nucleophilic substitution and reductive defluorination, which makes it an extraordinary precursor material for synthesis of large portfolio of graphene derivatives. Both chemical channels can be used to chemically manipulate with fluorographene and they can be tuned by suitable conditions, e.g., solvent.[17] In 2010 it was shown that fluorographene can be transformed to graphene by treatment with KI.[3] Nucleophiles can substitute the fluorine atoms and induce partial or full defluorination.[18] The fluorographene reactivity is triggered by point defects.[19] The knowledge on fluorographene reactivity can be used for synthesis of new graphene derivatives, which contain i) mixture of F and other functional groups (like, e.g., thiofluorographene containing both -F and -SH [20]) or ii) selectively only the functional group (and any -F groups). Alkyl and aryl groups can be selectively attached to graphene using Grignard reaction with fluorographene and this reaction leads to high-degree of graphene functionalization.[21] Very promising and selective graphene derivative cyanographene (graphene nitrile) was synthetized by reaction of NaCN with fluorographene. This material was further used for synthesis of graphene acid, i.e., graphene functionalized by -COOH groups over its surface, and it was shown that this graphene acid can be effectively conjugated with amines and alcohols. These findings open new door for high-yield and selective graphene functionalization.[22]
Other halogenated graphenes
Recent studies have also revealed that, similar to fluorination, full chlorination of graphene can be achieved. The resulting structure is called chlorographene.[23][24] However other theoretical calculations questioned stability of chlorographene under ambient conditions.[25]
Also graphene can be fluorinated or halofluorinated by CVD-method with fluorocarbons, hydro- or halofluorocarbons by heating while in contact of carbon material with fluoroorganic substance to form partially fluorinated carbons (so called Fluocar materials).[26][27]
An overview on preparation, reactivity and properties of halogenated graphenes in available in ACS Nano journal free of charge.[8]
See also
- "Chlorographene"
- Organofluorine chemistry
- Organofluorine compound
- Diamond
- Graphane
References
- Properties of Fluorinated Graphene Films Jeremy T. Robinson; James S. Burgess; Chad E. Junkermeier; Stefan C. Badescu; Thomas L. Reinecke; F. Keith Perkins; Maxim K. Zalalutdniov; Jeffrey W. Baldwin; James C. Culbertson; Paul E. Sheehan; Eric S. Snow (2010). "Properties of Fluorinated Graphene Films". Nano Letters. 10 (8): 3001–3005. Bibcode:2010NanoL..10.3001R. CiteSeerX 10.1.1.954.8747. doi:10.1021/nl101437p. PMID 20698613.
- Rahul R. Nair, Wencai Ren, Rashid Jalil, Ibtsam Riaz, Vasyl G. Kravets, Liam Britnell, Peter Blake, Fredrik Schedin, Alexander S. Mayorov, Shengjun Yuan, Mikhail I. Katsnelson, Hui-Ming Cheng, Wlodek Strupinski, Lyubov G. Bulusheva, Alexander V. Okotrub, Irina V. Grigorieva, Alexander N. Grigorenko, Kostya S. Novoselov, and Andre K. Geim (2010). "Fluorographene: A Two-Dimensional Counterpart of Teflon". Small. 6 (24): 2877–2884. arXiv:1006.3016. doi:10.1002/smll.201001555. PMID 21053339. S2CID 10022293.CS1 maint: multiple names: authors list (link)
- Radek Zboril; Frantisek Karlicky; A.B. Bourlinos; T.A. Steriotis; A.K. Stubos; V. Georgakilas; K. Safarova; D. Jancik; C. Trapalis; Michal Otyepka (2010). "Graphene Fluoride: A Stable Stoichiometric Graphene Derivative and its Chemical Conversion to Graphene". Small. 6 (24): 2885–2891. doi:10.1002/smll.201001401. PMC 3020323. PMID 21104801.
- Garcia, J. C.; de Lima, D. B.; Assali, L. V. C.; Justo, J. F. (2011). "Group IV graphene- and graphane-like nanosheets". J. Phys. Chem. C. 115 (27): 13242. arXiv:1204.2875. doi:10.1021/jp203657w. S2CID 98682200.
- Matus Dubecky; Eva Otyepkova; Petr Lazar; Frantisek Karlicky; Martin Petr; Klara Cepe; Pavel Banas; Radek Zboril; Michal Otyepka (2015). "Reactivity of Fluorographene: A Facile Way toward Graphene Derivatives". J. Phys. Chem. Lett. 6 (8): 1430–1434. doi:10.1021/acs.jpclett.5b00565. PMID 26263147.
- Withers, Freddie; Dubois, Marc; Savchenko, Alexander K. (2010). "Electron properties of fluorinated single-layer graphene transistors". Phys. Rev. B. 82 (7): 073403. arXiv:1005.3474. Bibcode:2010PhRvB..82g3403W. doi:10.1103/PhysRevB.82.073403. S2CID 119209248.
- Reversible fluorination of graphene: Evidence of a two-dimensional wide band gap semiconductor S.-H. Cheng, K. Zou, F. Okino, H. R. Gutierrez, A. Gupta, N. Shen, P. C. Eklund, J. O. Sofo, and J. Zhu Phys. Rev. B 2010; 81, 205435 doi:10.1103/PhysRevB.81.205435
- Karlicky F, Datta KKR, Otyepka M, Zboril R Halogenated Graphenes: Rapidly Growing Family of Graphene Derivatives. ACS Nano, 2013, 7 (8), pp 6434–6464 doi:10.1021/nn4024027
- Karlicky F, Otyepka M 'Band Gaps and Optical Spectra of Chlorographene, Fluorographene and Graphane from G0W0, GW0 and GW Calculations on Top of PBE and HSE06 Orbitals. J. Chem. Theory. Comput., 2013, 9 (9), doi:10.1021/ct400476r
- Artyukhov, V. I. and Chernozatonskii, L. A., Structure and Layer Interaction in Carbon Monofluoride and Graphane: A Comparative Computational Study. J. Phys. Chem. A, 2010, 114 (16), pp 5389–5396 doi:10.1021/jp1003566
- Leenaerts, O., Peelaers, H., Hernández-Nieves, A. D., Partoens, B. and Peeters, F. M., First-principles investigation of graphene fluoride and graphane. Phys. Rev. B 82, 195436 (2010) doi:10.1103/PhysRevB.82.195436
- Samarakoon, D. K., Chen, Z., Nicolas, C. and Wang, X.-Q. , Structural and Electronic Properties of Fluorographene. Small, n/a. doi:10.1002/smll.201002058
- Structural and Electronic Properties of Hybrid Fluorographene–Graphene Nanoribbons: Insight from First-Principles Calculations Shaobin Tang, Shiyong Zhang The Journal of Physical Chemistry C Article ASAP doi:10.1021/jp204880f
- Sahin H., Topsakal M., and Ciraci H., Structures of fluorinated graphene and their signatures, Physical Review B 83, 115432 (2011) doi:10.1103/PhysRevB.83.115432
- Garcia, J. C.; de Lima, D. B.; Assali, L. V. C.; Justo, J. F. (2011). "Group IV graphene- and graphane-like nanosheets". J. Phys. Chem. C. 115 (27): 13242. arXiv:1204.2875. doi:10.1021/jp203657w. S2CID 98682200.
- Ho, Kuan-I; Boutchich, Mohamed; Su, Ching-Yuan; Moreddu, Rosalia; Marianathan, Eugene Sebastian Raj; Montes, Laurent; Lai, Chao-Sung (2015). "A Self-Aligned High-Mobility Graphene Transistor: Decoupling the Channel with Fluorographene to Reduce Scattering". Advanced Materials. 27 (41): 6519–6525. doi:10.1002/adma.201502544. PMID 26398725.
- Matochová D, Medved M, Aristides B, Steklý T, Zbořil R, Otyepka M 2D Chemistry - Chemical Control of Graphene Derivatization. J. Phys. Chem. Lett., 2018, 9 (13), pp 3580–3585 . doi:10.1021/acs.jpclett.8b01596
- Dubecký M, Otyepková E, Lazar P, Karlický F, Petr M, Čépe K, Banáš P, Zbořil R, Otyepka M Reactivity of Fluorographene: A Facile Way toward Graphene Derivatives. J. Phys. Chem. Lett., 2015, 6 (8), pp 1430–1434 doi:10.1021/acs.jpclett.5b00565
- Medved M, Zoppellaro G, Ugolotti J, Matochová D, Lazar P, Pospíšil T, Bakandritsos A, Tuček J, Zbořil R, Otyepka M Reactivity of fluorographene is triggered by point defects: beyond the perfect 2D world. Nanoscale, 2018, 10, pp 4696-4707 doi:10.1039/C7NR09426DPMC 5892133
- Urbanová V, Holá K, Bourlinos AB, Čépe K, Ambrosi A, Loo AH, Pumera M, Karlický F, Otyepka M, Zbořil R Thiofluorographene-Hydrophilic Graphene Derivative with Semiconducting and Genosensing Properties. Adv. Mater., 2015, 27 (14), pp 2305–2310 doi:10.1002/adma.201500094
- Chronopoulos DD, Bakandritsos A, Lazar P, Pykal M, Čépe K, Zbořil R, Otyepka M High-Yield Alkylation and Arylation of Graphene via Grignard Reaction with Fluorographene. Chem. Mater., 2017, 29 (3), pp 926–930 doi:10.1021/acs.chemmater.6b05040
- Bakandritsos A, Pykal M, Blonski P, Jakubec P, Chronopoulos DD, Poláková K, Georgakilas V, Cepe K, Tomanec O, Ranc V, Bourlinos AB, Zbořil R, Otyepka M Cyanographene and Graphene Acid - Emerging Derivatives Enabling High-Yield and Selective Functionalization of Graphene. ACS Nano, 2017, 11 (3), pp 2982–2991 doi:10.1021/acsnano.6b08449
- Sahin, H (2012). "Chlorine Adsorption on Graphene: Chlorographene". The Journal of Physical Chemistry C. 116 (45): 24075–24083. arXiv:1211.5242. doi:10.1021/jp307006c. S2CID 44109838.
- Li, B (2011). "Photochemical Chlorination of Graphene". ACS Nano. 5 (7): 5957–61. doi:10.1021/nn201731t. PMID 21657242.
- Karlicky, F; et al. (2012). "Band gaps and structural properties of graphene halides and their derivates: A hybrid functional study with localized orbital basis sets". The Journal of Chemical Physics. 137 (3): 034709. arXiv:1209.4205. Bibcode:2012JChPh.137c4709K. doi:10.1063/1.4736998. PMID 22830726. S2CID 36374882.
- "United States Patent: 10000382 - Method for carbon materials surface modification by the fluorocarbons and derivatives".
- "WO16072959 Method for Carbon Materials Surface Modification by the Fluorocarbons and Derivatives". patentscope.wipo.int. Retrieved 2018-09-13.