Geosmin
Geosmin is an irregular sesquiterpene, produced from the universal sesquiterpene precursor farnesyl pyrophosphate (also known as farnesyl diphosphate), in a two-step Mg2+-dependent reaction.[1] Geosmin, along with the irregular monoterpene 2-methylisoborneol, together account for the majority of biologically-caused taste and odor outbreaks in drinking water worldwide.[2] Geosmin has a distinct earthy or musty odor, which most people can easily smell. The odor detection threshold of geosmin is very low, ranging from 0.006 to 0.01 micrograms per liter in water.[2] Geosmin is also responsible for the earthy taste of beetroots and a contributor to the strong scent (petrichor) that occurs in the air when rain falls after a dry spell of weather or when soil is disturbed.[3]
 | |
| Names | |
|---|---|
| IUPAC name
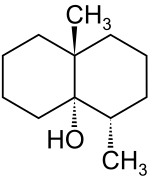
(4S,4aS,8aR)-4,8a-dimethyloctahydronaphthalen-4a(2H)-ol | |
| Other names
(4S,4aS,8aR)-4,8a-Dimethyl-1,2,3,4,5,6,7,8-octahydronaphthalen-4a-ol; 4,8a-Dimethyl-decahydronaphthalen-4a-ol; Octahydro-4,8a-dimethyl-4a(2H)-naphthalenol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.039.294 |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C12H22O | |
| Molar mass | 182.307 g·mol−1 |
| Boiling point | 270 to 271 °C (518 to 520 °F; 543 to 544 K) |
| Hazards | |
| Flash point | 104 °C (219 °F; 377 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
In chemical terms, it is a bicyclic alcohol with formula C12H22O, a derivative of decalin. Its name is derived from the Ancient Greek γεω- geō- "earth" and ὀσμή osmḗ "smell". The word was coined in 1965 by the American biochemist Nancy N. Gerber (1929–1985) and the French-American biologist Hubert A. Lechevalier (1926–2015).[4][5]
Production
Geosmin is produced by various blue-green algae (cyanobacteria) and filamentous bacteria in the class Actinomyces, and also some other prokaryotes and eukaryotes. The main genera in the cyanobacteria that have been shown to produce geosmin include Anabaena, Phormidium, and Planktothrix, while the main genus in the Actinomyces that produces geosmin is Streptomyces.[2][6][7][8] Communities whose water supplies depend on surface water can periodically experience episodes of unpleasant-tasting water when a sharp drop in the population of these bacteria releases geosmin into the local water supply. Under acidic conditions, geosmin decomposes into odorless substances.[4]
In 2006, the biosynthesis of geosmin by a bifunctional Streptomyces coelicolor enzyme was unveiled.[9][10] A single enzyme, geosmin synthase, converts farnesyl diphosphate to geosmin in a two-step reaction.
Not all blue-green algae cyanobacteria produce geosmin. Identification of species that might produce geosmin is traditionally done through microscopic identification of algae as geosmin producers, a technique that is labor-intensive and requires specialized knowledge. Recent advances in molecular biology have enabled identification of a geosmin synthase gene, geoA, which is present in cyanobacterial species that produce geosmin, but is not present in other cyanobacterial species.[11] Amplification of this gene from water samples using real-time PCR may permit predictions of taste and odor events caused by cyanobacteria in fresh water.
Effects
The human nose is extremely sensitive to geosmin and is able to detect it at concentrations as low as 5 parts per trillion.[12]
Geosmin is responsible for the muddy smell in many commercially important freshwater fish such as carp and catfish.[13][14] Geosmin combines with 2-methylisoborneol, which concentrates in the fatty skin and dark muscle tissues. It breaks down in acid conditions; hence, vinegar and other acidic ingredients are used in fish recipes to reduce the muddy flavor.[15] Taste and odor compounds including geosmin lead to an unpleasant taste of drinking water which is perceived by consumers as an indication of poor water quality.[16]
This compound is reported to be an issue for saltwater fish farmed in recirculating aquaculture systems, such as Atlantic salmon., but there are also studies that show that the presence in seawater is significantly lower than that found in freshwater which is why many people consider freshwater fish to taste muddy compared to marine fish. These systems rely on biological filtration using cultured microbial communities to process the nitrogenous waste from the fish (ammonia) into less harmful compounds (nitrite and nitrate) that can be tolerated at higher concentrations. However, geosmin-producing bacteria can also grow in these systems, and often require fish to be transferred to an additional "finishing" or "purge" system where they are not fed for several days prior to harvest to remove off-flavor compounds and empty the intestinal tract. This process is also known as depuration.
See also
References
- Watson, W.; Juttner, F. (2019). Taste and Odour in Source and Drinking Water: Causes, Controls, and Consequences. IWA Publishing. ISBN 9781780406657.
- "Biochemical and Ecological Control of Geosmin and 2-methylisoborneol in Source Waters". Applied and Environmental Microbiology. 73. July 2007.
- "The earth's perfume". Protein Spotlight (35). June 2003.
- Gerber, N. N.; Lechevalier, H. A. (November 1965). "Geosmin, an earthly-smelling substance isolated from actinomycetes". Applied Microbiology. 13 (6): 935–938. PMC 1058374. PMID 5866039.
- "geosmin". Merriam-Webster Dictionary.
- Izaguirre, G.; Taylor, W. D. (2004). "A guide to geosmin- and MIB-producing cyanobacteria in the United States". Water Science and Technology. 49 (9): 19–24. doi:10.2166/wst.2004.0524. PMID 15237602.
- Zaitlin, B.; Watson, S. B. (2006). "Actinomycetes in relation to taste and odour in drinking water: Myths, tenets and truths". Water Research. 40 (9): 1741–1753. doi:10.1016/j.watres.2006.02.024. PMID 16600325.
- Suurnäkki, S.; Gómez Sáez, G. V.; Rantala-Ylinen, A.; Jokela, J.; Fewer, D. P.; Sivonen, K. (2015). "Identification of geosmin and 2-methylisoborneol in cyanobacteria and molecular detection methods for the producers of these compounds". Water Research. 68 (1): 56–66. doi:10.1016/j.watres.2014.09.037. PMID 25462716.
- Jiang, J.; He, X.; Cane, D. E. (2006). "Geosmin biosynthesis. Streptomyces coelicolor germacradienol/germacrene D synthase converts farnesyl diphosphate to geosmin". Journal of the American Chemical Society. 128 (25): 8128–8129. doi:10.1021/ja062669x. PMID 16787064.
- Jiang, J.; He, X.; Cane, D. E. (2007). "Biosynthesis of the earthy odorant geosmin by a bifunctional Streptomyces coelicolor enzyme". Nature Chemical Biology. 3 (11): 711–715. doi:10.1038/nchembio.2007.29. PMC 3013058. PMID 17873868.
- Kutovaya, O.; Watson, S. (2014). "Development and application of a molecular assay to detect and monitor geosmin-producing cyanobacteria and actinomycetes in the Great Lakes". Journal of Great Lakes Research. 40 (2): 404–414. doi:10.1016/j.jglr.2014.03.016.
- Polak, E .H.; Provasi, J. (1992). "Odor sensitivity to geosmin enantiomers". Chemical Senses. 17: 23–26. doi:10.1093/chemse/17.1.23.
- Vallod, D.; Cravedi, J. P.; Hillenweck, A.; Robin, J. (April 2007). "Analysis of the off-flavor risk in carp production in ponds in Dombes and Forez (France)". Aquaculture International. 15 (3–4): 287–298. doi:10.1007/s10499-007-9080-7. ISSN 0967-6120.
- Lovell, R. T.; Lelana, I. Y.; Boyd, C. E.; Armstrong, M. S. (May 1986). "Geosmin and Musty-Muddy Flavors in Pond-Raised Channel Catfish". Transactions of the American Fisheries Society. 115 (3): 485–489. doi:10.1577/1548-8659(1986)115<485:gamfip>2.0.co;2. ISSN 0002-8487.
- Winkler, L. (2012). Westwood Lake Chronicles. Lawrence Winkler. ISBN 9780991694105.
- Bristow, R. L.; Young, I. S.; Pemberton, A.; Williams, J.; Maher, S. (2019). "An extensive review of the extraction techniques and detection methods for the taste and odour compound geosmin (trans-1,10-dimethyl-trans-9-decalol) in water". Trends in Analytical Chemistry. 110: 233–248. doi:10.1016/j.trac.2018.10.032.
Further reading
- Bear, I. J.; Thomas, R. G. (1964). "Nature of argillaceous odour". Nature. 201 (4923): 993–995. Bibcode:1964Natur.201..993B. doi:10.1038/201993a0.
- Bear, I. J.; Thomas, R. G. (1965). "Petrichor and plant growth". Nature. 207 (5005): 1415–1416. Bibcode:1965Natur.207.1415B. doi:10.1038/2071415a0.
