Hell–Volhard–Zelinsky halogenation
The Hell–Volhard–Zelinsky halogenation reaction halogenates carboxylic acids at the α carbon. The reaction is named after three chemists, the German chemists Carl Magnus von Hell (1849–1926) and Jacob Volhard (1834–1910) and the Russian chemist Nikolay Zelinsky (1861–1953).[1][2][3][4]
| Hell–Volhard–Zelinsky halogenation | |
|---|---|
| Named after | Carl Magnus von Hell Jacob Volhard Nikolay Zelinsky |
| Reaction type | Substitution reaction |
| Identifiers | |
| Organic Chemistry Portal | hell-volhard-zelinsky-reaction |
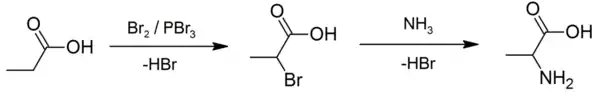
An example of the Hell–Volhard–Zelinsky reaction being used in practice can be seen in the preparation of alanine. An approach using a Strecker synthesis[5] was described as "excellent but tedious"[6] and so an alternative starting with propionic acid was developed. In its first step, a combination of bromine and phosphorus tribromide (catalyst) is used to prepare 2-bromopropanoic acid,[7] which is then converted to a racemic mixture of the amino acid product by ammonolysis.[6][8]
Mechanism
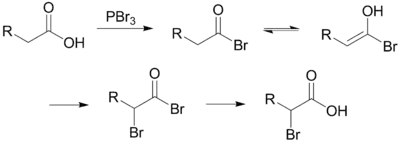
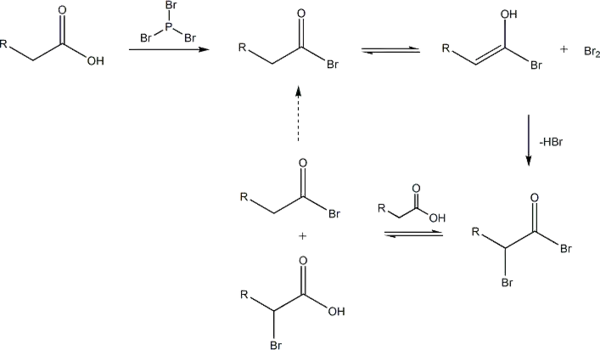
Unlike other halogenation reactions, this reaction takes place in the absence of a halogen carrier. The reaction is initiated by addition of a catalytic amount of PBr3, after which one molar equivalent of Br2 is added.
PBr3 replaces the carboxylic OH with a bromide, resulting in a carboxylic acid bromide. The acyl bromide can then tautomerize to an enol, which will readily react with the Br2 to brominate a second time at the α position.
In neutral to slightly acidic aqueous solution, hydrolysis of the α-bromo acyl bromide occurs spontaneously, yielding the α-bromo carboxylic acid in an example of a nucleophilic acyl substitution. If an aqueous solution is desirable, a full molar equivalent of PBr3 must be used as the catalytic chain is disrupted.
If little nucleophilic solvent is present, reaction of the α-bromo acyl bromide with the carboxylic acid yields the α-bromo carboxylic acid product and regenerates the acyl bromide intermediate. In practice a molar equivalent of PBr3 is often used anyway to overcome the slow reaction kinetics.
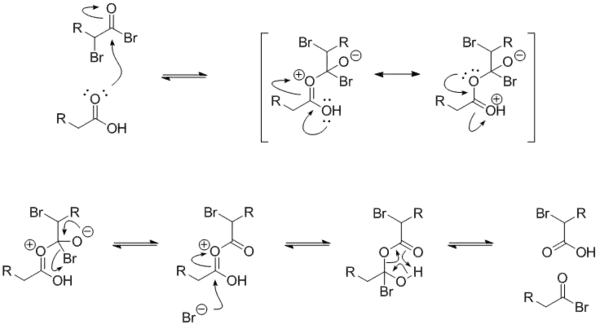
The mechanism for the exchange between an alkanoyl bromide and a carboxylic acid is below. The α-bromoalkanoyl bromide has a strongly electrophilic carbonyl carbon because of the electron-withdrawing effects of the two bromides.
See also
References
- von Hell, Carl Magnus (1881). "Ueber eine neue Bromirungsmethode organischer Säuren" [About a new bromination method for organic acids]. Berichte (in German). 14: 891–893. doi:10.1002/cber.188101401187.
- Volhard, Jacob (1887). "Ueber Darstellung α-bromirter Säuren" [On the Representation of α-Brominated Acids]. Annalen der Chemie (in German). 242 (1–2): 141–163. doi:10.1002/jlac.18872420107.
- Zelinsky, Nikolay (1887). "Ueber eine bequeme Darstellungsweise von α-Brompropionsäureester" [About a convenient representation of an ester of α-bromopropionic acid]. Berichte (in German). 20: 2026. doi:10.1002/cber.188702001452.
- Allen, C. Freeman; Kalm, Max J. (1958). "2-Methylenedodecanoic Acid". Organic Syntheses. 38: 47. doi:10.15227/orgsyn.038.0047.; Collective Volume, 4, p. 616
- Kendall, E. C.; McKenzie, B. F. (1929). "dl-Alanine". Organic Syntheses. 9: 4. doi:10.15227/orgsyn.009.0004.; Collective Volume, 1, p. 21
- Tobie, Walter C.; Ayres, Gilbert B. (1937). "Synthesis of d,l-Alanine in Improved Yield from α-Bromopropionic Acid and Aqueous Ammonia". Journal of the American Chemical Society. 59 (5): 950. doi:10.1021/ja01284a510.
- Marvel, C. S.; du Vigneaud, V. (1931). "α-Bromoisovaleric acid". Organic Syntheses. 11: 20. doi:10.15227/orgsyn.011.0020.; Collective Volume, 2, p. 93
- Tobie, Walter C.; Ayres, Gilbert B. (1941). "dl-Alanine". Organic Syntheses. doi:10.15227/orgsyn.009.0004.; Collective Volume, 1, p. 21