Hemiacetal
A hemiacetal or a hemiketal is a compound that results from the addition of an alcohol to an aldehyde or a ketone, respectively. The Greek word hèmi, meaning half, refers to the fact that a single alcohol has been added to the carbonyl group, in contrast to acetals or ketals, which are formed when a second alkoxy group has been added to the structure.[1]
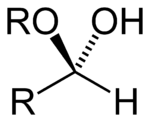
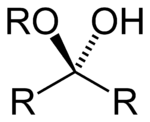
Formula and formation
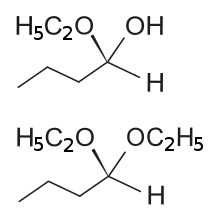
The general formula of a hemiacetal is R1R2C(OH)OR, where R1 or R2 is often hydrogen and R (bonded to O) is not hydrogen.
While in the IUPAC definition of a hemiacetal R1 or R2 may or may not be a hydrogen, in a hemiketal none of the R-groups can be a hydrogen. Hemiketals are regarded as hemiacetals where none of the R-groups are H, and are therefore a subclass of the hemiacetals.[2]
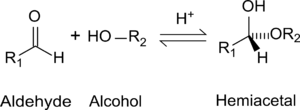 |
| Formation of hemiacetals |
 |
| Formation of hemiketals |
Cyclic hemiacetals and hemiketals
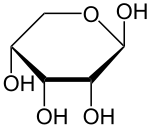 Ribopyranose | 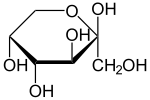 Fructopyranose |
| Left a lactol of ribose, a cyclic hemiacetal. Right a lactol of fructose, a cyclic hemiketal. | |
Hemiacetals and hemiketals are generally not stable compounds. In some cases however, stable cyclic hemiacetals and hemiketals, called lactols,[3] can be readily formed, especially with 5- and 6-membered rings. In this case an intramolecular OH group reacts with the carbonyl group. Glucose and many other aldoses exist as cyclic hemiacetals whereas fructose and similar ketoses exist as cyclic hemiketals.
Synthesis
Hemiacetals can be synthesized in a number of ways:
- Nucleophilic addition of an alcohol to a carbonyl group of an aldehyde
- Nucleophilic addition of an alcohol to a resonance stabilized hemiacetal cation
- Partial hydrolysis of an acetal
Reactions
Hemiacetals and hemiketals may be thought of as intermediates in the reaction between alcohols and aldehydes or ketones, with the final product being an acetal or a ketal:
- -C=O + 2 ROH ⇌ -C(OH)(OR) + ROH ⇌ -C(OR)2 + H2O
A hemiacetal can react with an alcohol under acidic conditions to form an acetal, and can dissociate to form an aldehyde and an alcohol.
An aldehyde dissolved in water exists in equilibrium with low concentrations of its hydrate, R-CH(OH)2. Similarly, in excess alcohol, the aldehyde, its hemiacetal, and its acetal all exist in solution.
A hemiacetal results from nucleophilic attack by the alcohol's hydroxyl group on the carbon of the C=O bond. Acetals are products of substitution reactions catalyzed by acid. The presence of acid improves the leaving capacity of the hydroxyl group and enables its substitution with an alkoxyl group (-OR). The conversion of a hemiacetal to an acetal is an SN1 reaction.
Ketones give hemiketals and ketals. These do not form as readily as hemiacetals and acetals. To increase yields of ketals or acetals, water formed during the reaction can be removed, in accord with Le Châtelier's principle.
References
- Fox, Marye Anne; Whitesell, James K. (2004). Organic Chemistry. Jones & Bartlett Learning. p. 590. ISBN 9780763721978.
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "hemiketals". doi:10.1351/goldbook.H02776
- IUPAC Gold Book lactols