Carbonyl group
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound.

The term carbonyl can also refer to carbon monoxide as a ligand in an inorganic or organometallic complex (a metal carbonyl, e.g. nickel carbonyl).
The remainder of this article concerns itself with the organic chemistry definition of carbonyl, where carbon and oxygen share a double bond.
Carbonyl compounds
A carbonyl group characterizes the following types of compounds:
| Compound | Aldehyde | Ketone | Carboxylic acid | Carboxylate ester | Amide |
| Structure | 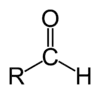 |  | 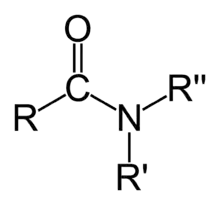 | ||
| General formula | RCHO | RCOR' | RCOOH | RCOOR' | RCONR'R'' |
| Compound | Enone | Acyl halide | Acid anhydride | Imide |
| Structure | 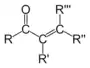 | 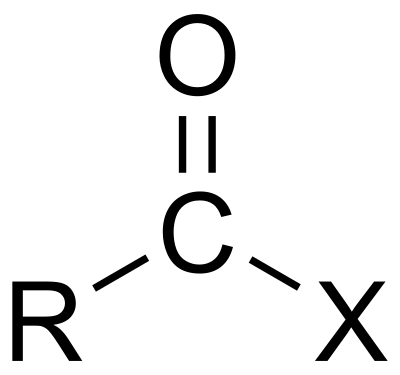 |  |  |
| General formula | RC(O)C(R')CR''R''' | RCOX | (RCO)2O | RC(O)N(R')C(O)R'' |

Other organic carbonyls are urea and the carbamates, the derivatives of acyl chlorides chloroformates and phosgene, carbonate esters, thioesters, lactones, lactams, hydroxamates, and isocyanates. Examples of inorganic carbonyl compounds are carbon dioxide and carbonyl sulfide.
A special group of carbonyl compounds are 1,3-dicarbonyl compounds that have acidic protons in the central methylene unit. Examples are Meldrum's acid, diethyl malonate and acetylacetone.
Reactivity
Carbonyl resonance chemistry
Because oxygen is more electronegative than carbon, carbonyl compounds often have resonance structures which affect their reactivity. This relative electronegativity draws electron density away from carbon, increasing the bond's polarity, therefore making carbon an electrophile (i.e. slightly positive). Carbon can then be attacked by nucleophiles (e.g. negatively charged ions, like the cyanide ion) or a negatively charged part of another molecule (e.g. the lone pair electrons of nitrogen in the ammonia molecule). During the reaction, the carbon-oxygen double bond is broken, and the carbonyl group may experience addition reactions. This reaction is known as addition-elimination (because a water molecule is often lost) or condensation.[1] The electronegative oxygen also can react with an electrophile; for example a proton in an acidic solution or with Lewis acids to form an oxocarbenium ion.

The polarity of oxygen also makes the alpha hydrogens of carbonyl compounds much more acidic (roughly 1030 times more acidic) than typical sp3 C-H bonds, such as those in methane. For example, the pKa values of acetaldehyde and acetone are 16.7 and 19 respectively,[2] while the pKa value of methane is extrapolated to be approximately 50.[3] This is because a carbonyl is in tautomeric resonance with an enol. The deprotonation of the enol with a strong base produces an enolate, which is a powerful nucleophile and can alkylate electrophiles such as other carbonyls.
Amides are the most stable of the carbonyl couplings due to their high resonance stabilization between the nitrogen-carbon and carbon-oxygen bonds.
Carbonyl reduction
Carbonyl groups can be reduced by reaction with hydride reagents such as NaBH4 and LiAlH4, with baker's yeast, or by catalytic hydrogenation. Ketones give secondary alcohols while aldehydes, esters and carboxylic acids give primary alcohols.
Carbonyl alkylation
Carbonyls can be alkylated in nucleophilic addition reactions using organometallic compounds such as organolithium reagents, Grignard reagents, or acetylides. Carbonyls also may be alkylated by enolates as in aldol reactions. Carbonyls are also the prototypical groups with vinylogous reactivity (e.g. the Michael reaction where an unsaturated carbon in conjugation with the carbonyl is alkylated instead of the carbonyl itself).
Carbonyl chemoselectivity
In case of multiple carbonyl types in one molecule, one can expect the most electrophilic carbonyl carbon to react first. Acyl chlorides and carboxylic anhydrides react fastest, followed by aldehydes and ketones. Esters react much more slowly and amides are almost completely unreactive due to resonance of the amide nitrogen towards the carbonyl group. This reactivity difference allows chemoselectivity when a reactant contains multiple carbonyl groups. An instructive example is found in the last part of the total synthesis of monensin by Kishi in 1979:[4]

The left-hand reactant possesses two potential electrophilic sites: an aldehyde (indicated in blue) and an ester (indicated in green). Only the aldehyde, which is more electrophilic, will react with the enolate of the methyl ketone in the other part of the molecule. The methyl ester remains untouched. Of course, other effects can play a role in this selectivity process, including electronic effects, steric effects, and thermodynamic versus kinetic reaction control.
Carbonyl specialty reactions
Other important reactions include:
- Carbonyl alpha-substitution reactions
- Wittig Reaction a phosphonium ylid is used to create an alkene
- Wolff-Kishner reduction into a hydrazone and further into a saturated alkane
- Clemmensen reduction into a saturated alkane
- Mozingo reduction into a saturated alkane
- Conversion into thioacetals
- Hydration to hemiacetals and hemiketals, and then to acetals and ketals
- Reaction with ammonia and primary amines to form imines
- Reaction with hydroxylamines to form oximes
- Reaction with cyanide anion to form cyanohydrins
- Oxidation with oxaziridines to acyloins
- Reaction with Tebbe's reagent and phosphonium ylides to alkenes.
- Perkin reaction, an aldol reaction variant
- Aldol condensation, a reaction between an enolate and a carbonyl
- Cannizzaro reaction, a disproportionation of aldehydes into alcohols and acids
- Tishchenko reaction, another disproportionation of aldehydes that gives a dimeric ester
- Nucleophilic abstraction is used to produce carbon dioxide
Spectroscopy
- Infrared spectroscopy: the C=O double bond absorbs infrared light at wavenumbers between approximately 1600–1900 cm−1(5263 nm to 6250 nm). The exact location of the absorption is well understood with respect to the geometry of the molecule. This absorption is known as the "carbonyl stretch" when displayed on an infrared absorption spectrum.[5] In addition, the ultraviolet-visible spectra of propanone in water gives an absorption of carbonyl at 257 nm.[6]
- Nuclear magnetic resonance: the C=O double-bond exhibits different resonances depending on surrounding atoms, generally a downfield shift. The 13C NMR of a carbonyl carbon is in the range of 160–220 ppm.
References
- "an introduction to aldehydes and ketones". www.chemguide.co.uk.
- Ouellette, R.J. and Rawn, J.D. "Organic Chemistry" 1st Ed. Prentice-Hall, Inc., 1996: New Jersey. ISBN 0-02-390171-3
- Claden, Johnathan; et al. Organic Chemistry. Oxford University Press. ISBN 978-0-19-850346-0.
- Nicolaou, Kyriacos Costa; E. J. Sorensen (1996). Classics in Total Synthesis: Targets, Strategies, Methods. Wiley-VCH. pp. 230–232. ISBN 3-527-29231-4.
- Mayo D.W., Miller F.A and Hannah R.W “Course Notes On The Interpretation of Infrared and Raman Spectra” 1st Ed. John Wiley & Sons Inc, 2004: New Jersey. ISBN 0-471-24823-1.
- "Archived copy" (PDF). Archived from the original (PDF) on 2015-08-24. Retrieved 2015-07-11.CS1 maint: archived copy as title (link)
Further reading
| Wikimedia Commons has media related to Carbonyl group. |
| Wikiquote has quotations related to: Carbonyl group |
- L.G. Wade, Jr. Organic Chemistry, 5th ed. Prentice Hall, 2002. ISBN 0-13-033832-X
- The Frostburg State University Chemistry Department. Organic Chemistry Help (2000).
- Advanced Chemistry Development, Inc. IUPAC Nomenclature of Organic Chemistry (1997).
- William Reusch. tara VirtualText of Organic Chemistry (2004).
- Purdue Chemistry Department (retrieved Sep 2006). Includes water solubility data.
- William Reusch. (2004) Aldehydes and Ketones Retrieved 23 May 2005.
- ILPI. (2005) The MSDS Hyperglossary- Anhydride.