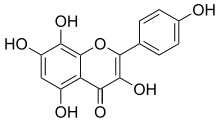
Herbacetin
 | |
 | |
| Names | |
|---|---|
| IUPAC name
3,5,7,8-tetrahydroxy-2-(4-hydroxyphenyl)chromen-4-one | |
| Other names
8-Hydroxykaempferol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.237.124 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H10O7 | |
| Molar mass | 302.238 g·mol−1 |
| Density | 1.799 g/mL |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Herbacetin is a flavonol, a type of flavonoid.
Glycosides
Herbacetin diglucoside can be isolated from flaxseed hulls.[1]
Rhodionin is a herbacetin rhamnoside found in Rhodiola species.[2]
Other related compounds
Rhodiolin, a flavonolignan, is the product of the oxidative coupling of coniferyl alcohol with the 7,8-dihydroxy grouping of herbacetin. It can be found in the rhizome of Rhodiola rosea.[3]
References
- Struijs, K.; Vincken, J. P.; Verhoef, R.; Van Oostveen-Van Casteren, W. H. M.; Voragen, A. G. J.; Gruppen, H. (2007). "The flavonoid herbacetin diglucoside as a constituent of the lignan macromolecule from flaxseed hulls". Phytochemistry. 68 (8): 1227–1235. doi:10.1016/j.phytochem.2006.10.022. PMID 17141814.
- Li, T.; Zhang, H. (2008). "Identification and Comparative Determination of Rhodionin in Traditional Tibetan Medicinal Plants of Fourteen Rhodiola Species by High-Performance Liquid Chromatography-Photodiode Array Detection and Electrospray Ionization-Mass Spectrometry". Chemical & Pharmaceutical Bulletin. 56 (6): 807–14. doi:10.1248/cpb.56.807. PMID 18520085.
- Zapesochnaya, G. G.; Kurkin, V. A. (1983). "The flavonoids of the rhizomes ofRhodiola rosea. II. A flavonolignan and glycosides of herbacetin". Chemistry of Natural Compounds. 19: 21–29. doi:10.1007/BF00579955. S2CID 7656479.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.