Hook effect
The hook effect or the prozone effect is an immunologic phenomenon whereby the effectiveness of antibodies to form immune complexes is sometimes impaired when concentrations of an antibody or an antigen are very high. The formation of immune complexes stops increasing with greater concentrations and then decreases with extremely high concentrations, producing a hook shape on a graph of measurements. There are versions of the hook effect with excess antibodies and versions with excess antigens. An important practical relevance of the phenomenon is as a type of interference that plagues certain immunoassays and nephelometric assays, resulting in false negatives or inaccurately low results. Other common forms of interference include antibody interference, cross-reactivity and signal interference. The phenomenon is caused by very high concentrations of a particular analyte or antibody and is most prevalent in one-step (sandwich) immunoassays.[2]
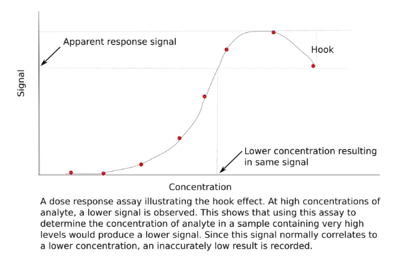
Mechanism and in vitro importance
Version with excess antibodies
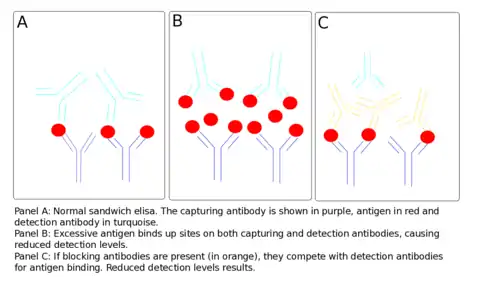
In an agglutination test, a person's serum (which contains antibodies) is added to a test tube, which contains a particular antigen. If the antibodies agglutinate with the antigen to form immune complexes, then the test is interpreted as positive. However, if too many antibodies are present that can bind to the antigen, then the antigenic sites are coated by antibodies, and few or no antibodies directed toward the pathogen are able to bind more than one antigenic particle.[3] Since the antibodies do not bridge between antigens, no agglutination occurs. Because no agglutination occurs, the test is interpreted as negative. In this case, the result is a false negative. The range of relatively high antibody concentrations within which no reaction occurs is called the prozone.[4]
Version with excess antigens
The effect can also occur because of antigen excess, when both the capture and detection antibodies become saturated by the high analyte concentration. In this case, no sandwich can be formed by the capturing antibody, the antigen and the detection antibody. In this case, free antigen is in competition with captured antigen for detection antibody binding.[5] Sequential addition of antigen and antibody, paired with stringent washing, can prevent the effect, as can increasing the relative concentration of antibody to antigen, thereby mediating the effect.
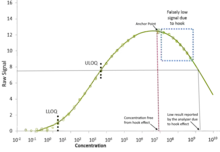
Examples include high levels of syphilis antibodies in HIV patients or high levels of cryptococcal antigen leading to false negative tests in undiluted samples.[6][7] This phenomenon is also seen in serological tests for Brucellosis. The serological test is mainly seen in the precipitation reaction. The antibody that fails to react is known as the blocking antibody and prevents the precipitating antibody from binding to the antigens. Thus the proper precipitation reaction does not take place. However, when the serum is diluted, the blocking antibody is as well and its concentration decreases enough for the proper precipitation reaction to occur.[8]
In vivo observations
Lewis Thomas described in his memoir a physiologic experiment of 1941 in which he observed the prozone effect in vivo: immunity in rabbits to meningococcus, which was robust, unexpectedly decreased when immunization was used to induce a heightened antibody response.[9] In other words, getting the rabbits' bodies to produce more antibodies against this bacterium had the counterproductive effect of decreasing their immunity to it. From the viewpoint of an overly simplistic notion of the antibody/antigen relationship, this seems paradoxical, although it is clearly logical from a viewpoint duly informed by present-day molecular biology. Thomas was interested in pursuing this physiologic research further, and remained so for decades afterward, but his career took him in other directions and he was not aware of anyone having pursued it by the time of his memoir.[9] One kind of relevance that he hypothesized for this in vivo blocking antibody concept was as a driver of human susceptibility to certain infectious diseases.[9] In the decades since, the concept has also been found to have clinical relevance in allergen immunotherapy, where blocking antibodies can interfere with other antibodies involved in hypersensitivity and thus improve allergy treatment.[10]
See also
References
- Schiettecatte, Johan; Anckaert, Ellen; Smitz, Johan (2012-03-23). "Interferences in Immunoassays". Advances in Immunoassay Technology. InTech. doi:10.5772/35797. ISBN 978-953-51-0440-7.
- Greenberg, G R; Jeejeebhoy, K N (1989-03-01). "Reply". Gut. 30 (3): 422–423. doi:10.1136/gut.30.3.422-a. ISSN 0017-5749. PMC 1378475.
- Miller, James (April 2004). "Interference in immunoassays: avoiding erroneous results" (PDF). CLI Online. CLI. Retrieved 3 February 2016.
- Gillet, Philippe; Mori, Marcella; Esbroeck, Marjan Van; Ende, Jef; Jacobs, Jan (2009-11-30). "Assessment of the prozone effect in malaria rapid diagnostic tests". Malaria Journal. 8 (1): 271. doi:10.1186/1475-2875-8-271. PMC 2789093. PMID 19948018.
- Tate, Jill; Ward, Greg (2004-05-01). "Interferences in Immunoassay". The Clinical Biochemist Reviews. 25 (2): 105–120. ISSN 0159-8090. PMC 1904417. PMID 18458713.
- Jurado RL, Campbell J, Martin PD (November 1993). "Prozone phenomenon in secondary syphilis. Has its time arrived?". Arch. Intern. Med. 153 (21): 2496–8. doi:10.1001/archinte.153.21.2496. PMID 7832818.
- Stamm AM, Polt SS (September 1980). "False-negative cryptococcal antigen test". JAMA. 244 (12): 1359. doi:10.1001/jama.244.12.1359. PMID 6997519.
- Cole, L. A.; Shahabi, S.; Butler, S. A.; Mitchell, H.; Newlands, E. S.; Behrman, H. R.; Verrill, H. L. (2001-02-01). "Utility of commonly used commercial human chorionic gonadotropin immunoassays in the diagnosis and management of trophoblastic diseases". Clinical Chemistry. 47 (2): 308–315. doi:10.1093/clinchem/47.2.308. ISSN 0009-9147. PMID 11159780.
- Thomas, Lewis (1983), The Youngest Science: Notes of a Medicine-Watcher
- Strait, RT; Morris, SC; Finkelman, FD (2006), "IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and Fc gamma RIIb cross-linking", J Clin Invest, 116 (3): 833–841, doi:10.1172/JCI25575, PMC 1378186, PMID 16498503