Test tube
A test tube, also known as a culture tube or sample tube, is a common piece of laboratory glassware consisting of a finger-like length of glass or clear plastic tubing, open at the top and closed at the bottom.
 Two small test tubes held in spring clamps | |
| Other names | Culture tube |
|---|---|
| Uses | Chemical reaction |
| Related items | Vacutainer Boiling tube Centrifuge tube |
Test tubes are usually placed in special-purpose racks.
Types and usage

Chemistry
Test tubes intended for general chemical work are usually made of glass, for its relative resistance to heat. Tubes made from expansion-resistant glasses, mostly borosilicate glass or fused quartz, can withstand high temperatures up to several hundred degrees Celsius.
Chemistry tubes are available in a multitude of lengths and widths, typically from 10 to 20 mm wide and 50 to 200 mm long.[1] The top often features a flared lip to aid pouring out the contents.
A chemistry test tube typically has a flat bottom, a round bottom, or a conical bottom. Some test tubes are made to accept a ground glass stopper or a screw cap. They are often provided with a small ground glass or white glaze area near the top for labelling with a pencil.
Test tubes are widely used by chemists to handle chemicals, especially for qualitative experiments and assays. Their spherical bottom and vertical sides reduce mass loss when pouring, make them easier to wash out, and allow convenient monitoring of the contents. The long, narrow neck of test tube slows down the spreading of gases to the environment.
Test tubes are convenient containers for heating small amounts of liquids or solids with a Bunsen burner or alcohol burner. The tube is usually held by its neck with a clamp or tongs. By tilting the tube, the bottom can be heated to hundreds of degrees in the flame, while the neck remains relatively cool, possibly allowing vapours to condense on its walls. A boiling tube is a large test tube intended specifically for boiling liquids.
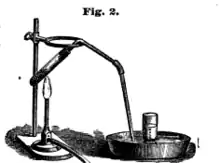
A test tube filled with water and upturned into a water-filled beaker is often used to capture gases, e.g. in electrolysis demonstrations.
A test tube with a stopper is often used for temporary storage of chemical or biological samples.
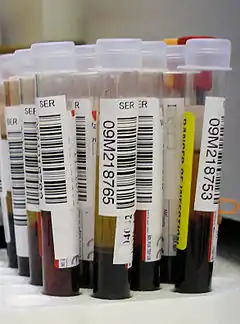
Biosciences
Culture tubes are test tubes used in biology and related sciences for handling and culturing all kinds of live organisms, such as molds, bacteria, seedlings, plant cuttings, etc.. Some racks for culture tubes are designed to hold the tubes in a nearly horizontal position, so as to maximize the surface of the culture medium inside.
Culture tubes for biology are usually made of clear plastic (such as polystyrene or polypropylene) by injection molding [2] and are often discarded after use. Plastic test tubes with a screwtop cap are often called "Falcon tubes" after a line manufactured by Becton Dickinson.[3]
Some sources consider that the presence of a lip is what distinguishes a test tube from a culture tube.[4]
Clinical medicine
In clinical medicine, sterile test tubes with air removed, called vacutainers, are used to collect and hold samples of physiological fluids such as blood, urine, pus, and synovial fluid. These tubes are commonly sealed with a rubber stopper and often have a specific additive placed in the tube with the stopper color indicating the additive. For example, a blue-top tube is a 5 ml test tube containing sodium citrate as an anticoagulant, used to collect blood for coagulation and glucose-6-phosphate dehydrogenase testing.[5] Small vials used in medicine may have a snap-top (also called a hinge cap) moulded as part of the vial.
| Tube cap color or type | Additive | Usage and comments |
|---|---|---|
| Blood culture bottle | Sodium polyanethol sulfonate (anticoagulant) and growth media for microorganisms | Usually drawn first for minimal risk of contamination.[6] Two bottles are typically collected in one blood draw; one for aerobic organisms and one for anaerobic organisms.[7] |
| Light blue | Sodium citrate (anticoagulant) | Coagulation tests such as prothrombin time (PT) and partial thromboplastin time (PTT) and thrombin time (TT). Tube must be filled 100%. |
| Plain red | No additive | Serum: Total complement activity, cryoglobulins |
| Gold | Clot activator and serum separating gel[8] | Serum-separating tube: Tube inversions promote clotting. Most chemistry, endocrine and serology tests, including hepatitis and HIV. |
| Dark green | Sodium heparin (anticoagulant) | Chromosome testing, ammonia, lactate, HLA typing |
| Mint green | Lithium heparin (anticoagulant) | Plasma. Tube inversions prevent clotting |
| Lavender ("purple") | EDTA (chelator / anticoagulant) | Whole blood: CBC, ESR, blood levels of tacrolimus and cyclosporin, platelet antibodies, Coombs test, flow cytometry |
| Pink | EDTA (chelator / anticoagulant) | Blood typing and cross-matching, direct Coombs test, HIV viral load |
| Royal blue | EDTA (chelator / anticoagulant) | Trace elements, heavy metals, most drug levels, toxicology |
| Tan | EDTA (chelator / anticoagulant) | Lead |
| Gray |
|
Glucose, lactate[10] |
| Yellow | Acid-citrate-dextrose A (anticoagulant) | Tissue typing, DNA studies, HIV cultures |
| Pearl ("white") | Separating gel and (K2)EDTA | PCR for adenovirus, toxoplasma and HHV-6 |
Other uses
Test tubes are sometimes put to casual uses outside of lab environments, e.g. as flower vases, glassware for certain weak shots, or containers for spices. They can also be used for raising queen ants during their first months of development.
Variants
Boiling tube
A boiling tube is a small cylindrical vessel used to strongly heat substances in the flame of a Bunsen burner. A boiling tube is essentially a scaled-up test tube, being about 50% larger.
They are designed to be wide enough to allow substances to boil violently as opposed to a test tube, which is too narrow; a boiling liquid can explode out of the end of test tubes when they are heated, as there is no room for bubbles of gas to escape independently of the surrounding liquid. This phenomenon is called bumping.
Being large and thick-walled, It is primarily used to hold small quantities of substances which are undergoing direct heating by a Bunsen burner or other heat source.[11] This type of tube is used in the sodium fusion test.
Ignition tubes are often difficult to clean due to the small bore. When used to heat substances strongly, some char may stick to the walls as well. They are usually disposable.
See also

References
- MiniScience.com catalog: Test Tube, accessed March 27, 2009.
- M. Jeremy Ashcraft; General Manager; Lake Charles Manufacturing (2007). Test Tube Molding Process: A discussion on the molding of plastic test tubes. Lake Charles Manufacturing.
- "BD Falcon Tubes and Pipets" (PDF). Becton Dickinson. Retrieved 14 March 2016.
- Thomas Scott (transl., 1996), Concise Encyclopedia: Biology. Walter de Gruyter. ISBN 3-11-010661-2, ISBN 978-3-11-010661-9. 1287 pages.
- TheFreeDictionary > blue top tube. Citing: McGraw-Hill Concise Dictionary of Modern Medicine. 2002 by The McGraw-Hill Companies, Inc.
- Pagana, KD; Pagana, TJ; Pagana, TN (19 September 2014). Mosby's Diagnostic and Laboratory Test Reference - E-Book. Elsevier Health Sciences. p. xiii. ISBN 978-0-323-22592-2.
- "Chapter 3.4.1: Blood cultures; general detection and interpretation". Clinical Microbiology Procedures Handbook. Wiley. 6 August 2020. ISBN 978-1-55581-881-4.
- "Specimen requirements/containers". Pathology & Laboratory Medicine, UCI School of Medicine. Retrieved 2020-09-10.
- Castellini MA, Castellini JM, Kirby VL (1992). "Effects of standard anticoagulants and storage procedures on plasma glucose values in seals". J Am Vet Med Assoc. 201 (1): 145–8. PMID 1644639.
- Amitava Dasgupta; Jorge L. Sepulveda (20 July 2019). Accurate Results in the Clinical Laboratory: A Guide to Error Detection and Correction. Elsevier Science. p. 131. ISBN 978-0-12-813777-2.
- Nichols, William Ripley (1877). An Elementary Manual of Chemistry: Abridged from Eliot and Storer's Manual, with the Co-operation of the Authors. Ivison, Blakeman, Taylor.
| Look up test tube in Wiktionary, the free dictionary. |
| Wikimedia Commons has media related to Test tubes. |
