Inhaler
An inhaler (also known as a puffer, pump or allergy spray) is a medical device used for delivering medicines into the lungs through the work of a person's breathing. This allows medicines to be delivered to and absorbed in the lungs, which provides the ability for targeted medical treatment to this specific region of the body, as well as a reduction in the side effects of oral medications. There are a wide variety of inhalers, and they are commonly used to treat numerous medical conditions with asthma and chronic obstructive pulmonary disease (COPD) being among the most notable.[1]
| Inhaler | |
|---|---|
 Metered-dose inhaler (MDI) | |
| Specialty | pulmonology |
Some of the common types of inhalers include meter-dosed inhalers, dry powder inhalers, soft mist inhalers, and nebulizers. Each device has advantages and disadvantages and can be selected based on specific patient needs, as well as age, coordination, and lung function.[2] Proper education on inhaler use is important to ensure that inhaled medication takes its proper effects in the lungs.[3]
Medical Uses
Inhalers are designed to deliver medication directly to the lungs through a person's own breathing. This may benefit a patient by providing medicines directly to areas of disease, allowing medication to take a greater effect on its intended target, and limit side effects of medications due to localized treatment.[1] Inhalers are used in a variety of different medical conditions with diseases of the lungs and respiratory system being among the most common. These conditions utilize medications designed to decrease airway inflammation and obstruction to allow for easier and less strained breathing.[4] Antibiotic medications have even been developed for inhalers to allow for direct delivery to areas of infection within the lungs.[5] Two of the most common conditions that warrant inhaler therapy are asthma and chronic obstructive pulmonary disease.[4][6]
Asthma
Asthma is a condition of intermittent airway obstruction due to inflammatory processes in the lungs. Inhaled medications are used to calm down the inflammation present in the lungs and allow for relief of the airway obstruction. Common inhaled medications used for treatment of asthma are salbutamol, corticosteroids, and salmeterol. These medications allow for patients to have relief of airway obstruction symptoms and reduced inflammation.[4]
Chronic Obstructive Pulmonary Disease (COPD)
COPD is an obstructive lung disease due to long-term damage to the airways of the lung. The long-term damage leads to the inability of the airways to open properly, causing airway obstruction. Inhaled medications allow for patient's to see improvement in symptoms and better function of daily living. Some commonly used inhaled medications in patient's with COPD are ipratroprium, salmeterol, and corticosteroids.[6]
Types
Meter-dosed Inhalers (MDI)
The most common type of inhaler is the pressurized metered-dose inhaler (MDI) which is made up of 3 standard components- a metal canister, plastic actuator, and a metering valve. The medication is typically stored in solution in a pressurized canister that contains a propellant or suspension. The MDI canister is attached to a plastic, hand-operated actuator. On activation, the metered-dose inhaler releases a fixed dose of medication in aerosol form through the actuator and into a patient's lungs.[7] These devices require significant coordination as a person must discharge the medication at or near the same time that they inhale in order for the medication to be effective.[8]

Dry Powder Inhalers (DPI)
Dry powder inhalers release a metered or device-measured dose of powdered medication that is inhaled through a DPI device. This device usually contains a chamber in which the powdered medication is deposited prior to each dosage.[3] The powder can then be inhaled with a quick breath.[1] This allows for medication to be delivered to the lungs without the need for use of propellant/suspension.[8]
Soft Mist Inhalers (SMI)
.png.webp)
Soft mist inhalers release a light mist containing medication without the need for a propellant/suspension. Upon pressing a button, the inhaler creates a mist of medication, allowing for inhalation into the lungs. SMIs suspend inhaled medications for roughly 1.2 seconds, which is longer than the average MDI inhaler suspension time period. This requires less coordination when using and may be helpful for young patients or patients that find the MDI inhalers difficult to use.[8]
Nebulizers
Nebulizers are designed to deliver medications over an extended period of time over multiple breaths through a mouthpiece or face mask. They generate a continuous mist with aerosolized medication, allowing a patient to breathe normally and receive medications.[8] They are commonly used in infants and toddlers requiring inhaled medications or in patients in the hospital who require inhaled medications.[2]
Propellants
In 2009, the FDA banned the use of inhalers that use chlorofluorocarbons (CFC) as propellants. In their place, inhalers now use hydrofluoroalkane (HFA). HFA is not environmentally inert as it is a greenhouse gas but it does not affect the ozone layer.[9] While some asthma sufferers and advocacy groups contend that HFA inhalers are not as effective,[10] published clinical studies indicate CFC and HFA inhalers are equally effective in controlling asthma.[11]
While the impact of CFCs from inhalers on the ozone layer had been minuscule (dwarfed by industrial processes using CFCs,) the FDA in its interpretation of the Montreal Protocol mandated the switch in propellants.[9] Patients expressed concern about the high price of the HFA inhalers as there are no generic versions, whereas generic CFC inhalers had been available.[10]
Proper Use
It is important to use proper technique when administering inhalers to self or others. Improper use of inhalers is very common and can lead to distribution of the medicine into the mouth or throat where it cannot take its desired effect.[1][12] Education on the correct use of inhalers for delivery of medications is a commonly cited topic in medical studies and a great deal of thought has been put into how best to help people learn to use their inhalers effectively.[13][3] Below is a description of proper inhaler technique for each different type of inhaler as well as a helpful video explaining what the text states.
Meter-dosed Inhalers:
1. Remove mouthpiece and shake inhaler for 5–10 seconds
2. Grip inhaler with mouthpiece on the bottom and canister on top. A finger should be placed on the canister to allow for delivery of medicine.
3. Breathe out completely and place mouth over mouthpiece.
4. As you begin to breathe in, press down on the canister (this releases the medicine).
5. Continue to breathe in slowly and deeply and hold your breath for 5–10 seconds (this keeps the medicine in your lungs where it is supposed to be).
6. Breathe out. If you are supposed to take multiple puffs of medicine, wait 15–30 seconds and repeat steps 1-5.
7. Replace mouthpiece[1]
With Spacer:
Place spacer at the mouthpiece of your meter-dosed inhaler and your mouth at the end of the spacer. Press down on the canister and breathe in deeply when ready for delivery of the medicine to the lungs. This decreases need for coordination of breathing with inhaler activation.[1]
Dry Powder Inhalers:
1. Prepare inhaler medication chamber (this will be different based on the type of inhaler but will involve preparing and opening the chamber with the medication)
2. Hold the inhaler with the chamber pointing towards you and breathe out completely with her head turned away from the inhaler
3. Place mouth over the chamber and take a quick, deep breath to allow medication to dispense in the lungs
4. Hold breath for 5–10 seconds and then exhale slowly
5. Repeat steps 1-4 if another dose is needed[1]
Soft Mist Inhalers:
1. Prime inhaler (this involves loading the cartridge and discharging the inhaler until a fine mist is visible - more explanation in the video)
2. Breathe out completely and place mouth around the mouthpiece while leaving space for the small holes on the side of the mouthpiece
3. Breathe in slowly while at the same time pressing the button to release the medication
4. Hold breath for 5–10 seconds
5. Breathe out slowly and repeat steps 1-4 if another dose of medication is required
If inhaler is used everyday, you should only have to prime the inhaler the first time using a new cartridge, but it may need to be primed again if it has not been used in multiple days.[1]
After use:
If using inhaled corticosteroids, rinse mouth out directly after use of inhaler. This helps to prevent infection.[1]
Nebulizer:
1. Place mouth over mouthpiece or face mask over nose and mouth
2. Turn on nebulizer machine
3. Breath normally for 10-20 min (or time allotted for treatment)
4. Turn off machine and remove face mask/mouthpiece[1]
History

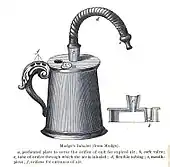
The idea of directly delivering medication into the lungs was based on ancient traditional cures that involved the use of aromatic and medicinal vapours. These did not involve any special devices beyond the apparatus used for burning or heating to produce fumes. Early inhalation devices included one devised by John Mudge in 1778. It had a pewter mug with a hole allowing attachment of a flexible tube. Mudge used it for the treatment of coughs using opium. These devices evolved with modifications by Wolfe, Mackenzie (1872) and better mouth attachments such as by Beigel in 1866. Many of these early inhalers needed heat to vapourize the active chemical ingredient. The benefits of forced expiration and inspiration to treat asthma were noted by J. S. Monell in 1865. Chemicals used in inhalers included ammonia, chlorine, iodine, tar, balsams, turpentine camphor and numerous others in combinations.[14] Julius Mount Bleyer used a variation in 1890 in New York.[15]

In 1968, Robert Wexler of Abbott Laboratories developed the Analgizer, a disposable inhaler that allowed the self-administration of methoxyflurane vapor in air for analgesia.[16] The Analgizer consisted of a polyethylene cylinder 5 inches long and 1 inch in diameter with a 1 inch long mouthpiece. The device contained a rolled wick of polypropylene felt which held 15 milliliters of methoxyflurane.
Because of the simplicity of the Analgizer and the pharmacological characteristics of methoxyflurane, it was easy for patients to self-administer the drug and rapidly achieve a level of conscious analgesia which could be maintained and adjusted as necessary over a period of time lasting from a few minutes to several hours. The 15 milliliter supply of methoxyflurane would typically last for two to three hours, during which time the user would often be partly amnesic to the sense of pain; the device could be refilled if necessary.[17]
The Analgizer was found to be safe, effective, and simple to administer in obstetric patients during childbirth, as well as for patients with bone fractures and joint dislocations,[17] and for dressing changes on burn patients.[18] When used for labor analgesia, the Analgizer allows labor to progress normally and with no apparent adverse effect on Apgar scores.[17] All vital signs remain normal in obstetric patients, newborns, and injured patients.[17] The Analgizer was widely utilized for analgesia and sedation until the early 1970s, in a manner that foreshadowed the patient-controlled analgesia infusion pumps of today.[19][20][21][22] The Analgizer inhaler was withdrawn in 1974, but use of methoxyflurane as a sedative and analgesic continues in Australia and New Zealand in the form of the Penthrox inhaler.[23][24][25][26][27][28]
See also
References
- National Asthma Education and Prevention, Program. (November 2007). "Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007". The Journal of Allergy and Clinical Immunology. 120 (5 Suppl): S94-138. doi:10.1016/j.jaci.2007.09.043. PMID 17983880.
- DePietro, Michael; Gilbert, Ileen; Millette, Lauren A.; Riebe, Michael (January 2018). "Inhalation device options for the management of chronic obstructive pulmonary disease". Postgraduate Medicine. 130 (1): 83–97. doi:10.1080/00325481.2018.1399042. ISSN 1941-9260. PMID 29210318. S2CID 705448.
- Maricoto, T; Monteiro, L; Gama, JMR; Correia-de-Sousa, J; Taborda-Barata, L (January 2019). "Inhaler Technique Education and Exacerbation Risk in Older Adults with Asthma or Chronic Obstructive Pulmonary Disease: A Meta-Analysis". Journal of the American Geriatrics Society. 67 (1): 57–66. doi:10.1111/jgs.15602. PMID 30291745.
- Rothe, Thomas; Spagnolo, Paolo; Bridevaux, Pierre-Olivier; Clarenbach, Christian; Eich-Wanger, Christine; Meyer, Franca; Miedinger, David; Möller, Alexander; Nicod, Laurent P.; Nicolet-Chatelain, Geneviève; Sauty, Alain (2018). "Diagnosis and Management of Asthma - The Swiss Guidelines". Respiration; International Review of Thoracic Diseases. 95 (5): 364–380. doi:10.1159/000486797. ISSN 1423-0356. PMID 29614508.
- Vardakas, Konstantinos Z.; Voulgaris, Georgios L.; Samonis, George; Falagas, Matthew E. (January 2018). "Inhaled colistin monotherapy for respiratory tract infections in adults without cystic fibrosis: a systematic review and meta-analysis". International Journal of Antimicrobial Agents. 51 (1): 1–9. doi:10.1016/j.ijantimicag.2017.05.016. ISSN 1872-7913. PMID 28669836.
- Stolz, Daiana; Barandun, Jürg; Borer, Heinz; Bridevaux, Pierre-Olivier; Brun, Patrick; Brutsche, Martin; Clarenbach, Christian; Eich, Christine; Fiechter, René; Frey, Martin; Geiser, Thomas (2018). "Diagnosis, Prevention and Treatment of Stable COPD and Acute Exacerbations of COPD: The Swiss Recommendations 2018" (PDF). Respiration; International Review of Thoracic Diseases. 96 (4): 382–398. doi:10.1159/000490551. ISSN 1423-0356. PMID 30138943. S2CID 52074520.
- Hickey, A.J., ed. (2004). Pharmaceutical Inhalation Aerosol Technology (2nd ed.). NY: Marcel Dekker.
- Navaie, Maryam; Dembek, Carole; Cho-Reyes, Soojin; Yeh, Karen; Celli, Bartolome R. (January 2020). "Device use errors with soft mist inhalers: A global systematic literature review and meta-analysis". Chronic Respiratory Disease. 17: 1479973119901234. doi:10.1177/1479973119901234. ISSN 1479-9731. PMC 6985977. PMID 31984767.
- Nick Baumann (July–August 2011). "Why You're Paying More to Breathe". Mother Jones.
- "Asthma Group Concerned "Green" Inhalers May Not be as Effective | ksdk.com | St. Louis, MO". ksdk.com. Retrieved 2010-11-21.
- Hendeles L, Colice GL, Meyer RJ (March 2007). "Withdrawal of albuterol inhalers containing chlorofluorocarbon propellants". N. Engl. J. Med. 356 (13): 1344–51. doi:10.1056/NEJMra050380. PMID 17392304.
- Cho-Reyes, Soojin; Celli, Bartolome R.; Dembek, Carole; Yeh, Karen; Navaie, Maryam (2019-07-24). "Inhalation Technique Errors with Metered-Dose Inhalers Among Patients with Obstructive Lung Diseases: A Systematic Review and Meta-Analysis of U.S. Studies". Chronic Obstructive Pulmonary Diseases (Miami, Fla.). 6 (3): 267–280. doi:10.15326/jcopdf.6.3.2018.0168. ISSN 2372-952X. PMC 6872219. PMID 31342732.
- Harris, K; Kneale, D; Lasserson, TJ; McDonald, VM; Grigg, J; Thomas, J (28 January 2019). "School-based self-management interventions for asthma in children and adolescents: a mixed methods systematic review". The Cochrane Database of Systematic Reviews. 1: CD011651. doi:10.1002/14651858.CD011651.pub2. PMC 6353176. PMID 30687940.
- Cohen, J. Solis (1876). Inhalation in the treatment of disease: its therapeutics and practice. Philadelphia: Lindsay & Blakiston.
- Bleyer, J. Mount (1890). "A new method of larygeal and bronchial medication by means of a spray and tube during the act of deep inspiration. Read in the Section of Laryngology and Otology at the Forty-first Annual Meeting of the American Medical Association, Nashville, Tenn., May, 1890". Journal of the American Medical Association. 15 (18): 634–636. doi:10.1001/jama.1890.02410440006001a.
- Wexler RE (1968). "Analgizer: Inhaler for supervised self-administration of inhalation anesthesia". Abbott Park, Illinois: Abbott Laboratories. Retrieved 2010-11-21. Cite journal requires
|journal=(help) - Romagnoli A, Busque L, Power DJ (1970). "The "analgizer" in a general hospital: a preliminary report". Canadian Journal of Anesthesia. 17 (3): 275–8. doi:10.1007/BF03004607. PMID 5512851.
- Packer KJ, Titel JH (1969). "Methoxyflurane analgesia for burns dressings: experience with the Analgizer (subscription required)". British Journal of Anaesthesia. 41 (12): 1080–5. CiteSeerX 10.1.1.1028.6601. doi:10.1093/bja/41.12.1080. PMID 4903969.
- Major V, Rosen M, Mushin WW (1966). "Methoxyflurane as an obstetric analgesic: a comparison with trichloroethylene". BMJ. 2 (5529): 1554–61. doi:10.1136/bmj.2.5529.1554. PMC 1944957. PMID 5926260.
- Dragon A, Goldstein I (1967). "Methoxyflurane: preliminary report on analgesic and mood modifying properties in dentistry (subscription required)". Journal of the American Dental Association. 75 (5): 1176–81. doi:10.14219/jada.archive.1967.0358. PMID 5233333.
- Firn S (1972). "Methoxyflurane analgesia for burns dressings and other painful ward procedures in children (subscription required)". British Journal of Anaesthesia. 44 (5): 517–22. doi:10.1093/bja/44.5.517. PMID 5044082.
- Josephson CA, Schwartz W (1974). "The Cardiff Inhaler and Penthrane. A method of sedation analgesia in routine dentistry". Journal of the Dental Association of South Africa. 29 (2): 77–80. PMID 4534883.
- Babl F, Barnett P, Palmer G, Oakley E, Davidson A (2007). "A pilot study of inhaled methoxyflurane for procedural analgesia in children (subscription required)". Pediatric Anesthesia. 17 (2): 148–53. doi:10.1111/j.1460-9592.2006.02037.x. PMID 17238886.
- Grindlay J, Babl FE (2009). "Efficacy and safety of methoxyflurane analgesia in the emergency department and prehospital setting". Emergency Medicine Australasia. 21 (1): 4–11. doi:10.1111/j.1742-6723.2009.01153.x. PMID 19254307. S2CID 40158248.
- Babl FE, Jamison SR, Spicer M, Bernard S (2006). "Inhaled methoxyflurane as a prehospital analgesic in children (subscription required)". Emergency Medicine Australasia. 18 (4): 404–10. doi:10.1111/j.1742-6723.2006.00874.x. PMID 16842312. S2CID 1619160.
- McLennan JV (2007). "Is methoxyflurane a suitable battlefield analgesic?" (PDF). Journal of the Royal Army Medical Corps. 153 (2): 111–3. doi:10.1136/jramc-153-02-08. PMID 17896540. S2CID 38517296. Archived from the original (PDF) on 2011-07-15.
- Medical Developments International Pty. Ltd. (2009). "PENTHROX (methoxyflurane) Inhalation: Product Information" (PDF). Springvale, Victoria, Australia: Medical Developments International Limited. Retrieved 2010-11-21.
- National Prescribing Service (2010). "Methoxyflurane (Penthrox) for analgesia (doctor's bag listing)" (PDF). NPS RADAR. Canberra, Australia: National Prescribing Service, Department of Health and Ageing. Retrieved 2010-11-21.
Further reading
- Patton J (February 1998). "Breathing life into protein drugs — Inhalation of therapeutic macromolecules is a feasible, natural, more people-friendly, delivery system". Nat. Biotechnol. 16 (2): 141–3. doi:10.1038/nbt0198-141. PMID 9487516. S2CID 20224465.
-solution.jpg.webp)