Kornblum oxidation
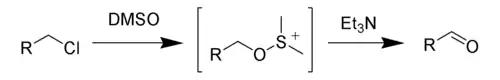
The Kornblum oxidation, named after Nathan Kornblum, is a chemical reaction of a primary halide with dimethyl sulfoxide (DMSO) to form an aldehyde.[1][2][3]
| Kornblum oxidation | |
|---|---|
| Named after | Nathan Kornblum |
| Reaction type | Organic redox reaction |
Mechanism
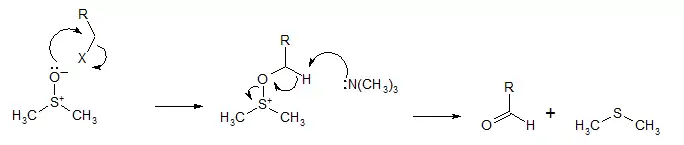
Like all DMSO-based oxidations, the Kornblum oxidation creates an alkoxysulfonium ion, which, in the presence of a base, such as triethylamine (Et3N), will eliminate to form the desired aldehyde.
Alterations
There are a number of variants and alternatives of the Kornblum oxidation. These alterations include using silver-assisted DMSO oxidations, the use of amine oxides as oxidants (occasionally called the Ganem oxidation), the use of pyridine-N-oxide or 2-picoline-N-oxide and a base, the use of metal nitrates, Sommelet oxidation, and Kröhnke oxidation. [4] The Kornblum oxidation can also be effected through the use of microwave assistance. Microwave assisted organic synthesis of the Kornblum oxidation has been shown to increase yield and decrease the reaction time through elimination of an unnecessary intermediate. The microwaves cause the solvent to reach its boiling point much sooner, possibly leading to evaporation of the dimethyl sulfide, therefore reducing any side products of the starting material reacting with the thioether. In usual heating, some aliphatic chlorides give low yields or no results at all, but with microwave assisted radiation, they can give intermediate yields.
References
- Kornblum, N.; Jones, W. J.; Anderson, G. J. (1959). "A New and Selective Method of Oxidation. The Conversion of Alkyl Halides and Alkyl Tosylates to Aldehydes". Journal of the American Chemical Society. 81 (15): 4113–4114. doi:10.1021/ja01524a080.
- Kornblum, N.; Powers, J. W.; Anderson, G. J.; Jones, W. J.; Larson, H. O.; Levand, O.; Weaver, W. M. (1957). "A New and Selective Method of Oxidation". Journal of the American Chemical Society. 79 (24): 6562. doi:10.1021/ja01581a057.
- Dave, Paritosh; Byun, Hoe-Sup; Engel, Robert (1986). "An Improved Direct Oxidation of Alkyl Halides to Aldehydes". Synthetic Communications. 16 (11): 1343–1346. doi:10.1080/00397918608056381.
- Kürti, László, and Barbara Czakó. (2005). Strategic Applications of Named Reactions in Organic Synthesis: Background and Detailed Mechanisms. Elsevier Academic Press. p. 250. ISBN 0124297854.CS1 maint: uses authors parameter (link)