Lepidodendrales
Lepidodendrales (from the Greek for "scale tree") were primitive, vascular, arborescent (tree-like) plants related to the lycopsids (club mosses). Members of Lepidodendrales are the best understood of the fossil lycopsids due to the vast diversity of Lepidodendrales specimens and the diversity in which they were preserved; the extensive distribution of Lepidodendrales specimens as well as their well-preservedness lends paleobotanists exceptionally detailed knowledge of the coal-swamp giants’ reproductive biology, vegetative development, and role in their paleoecosystem. The defining characteristics of the Lepidodendrales are their secondary xylem, extensive periderm development, three-zoned cortex, rootlike appendages known as stigmarian rootlets arranged in a spiralling pattern, and megasporangium each containing a single functional megaspore that germinates inside the sporangium. Many of these different plant organs have been assigned both generic and specific names as relatively few have been found organically attached to each other. Some specimens have been discovered which indicate heights of 40[1] and even 50 meters[2] and diameters of over 2 meters at the base. The massive trunks of some species branched profusely, producing large crowns of leafy twigs; though some leaves were up to 1 meter long, most were much shorter, and when leaves dropped from branches their conspicuous leaf bases remained on the surface of branches. Strobili could be found at the tips of distal branches or in an area at the top of the main trunk. The underground organs of Lepidodendrales typically consisted of dichotomizing axes bearing helically arranged, lateral appendages serving an equivalent function to roots. Sometimes called "giant club mosses", they are in fact more closely related to quillworts than to club mosses.
| Lepidodendrales Temporal range: Carboniferous to Triassic | |
|---|---|
 | |
| A bifurcation in Lepidophloios | |
| Scientific classification | |
| Kingdom: | Plantae |
| Clade: | Tracheophytes |
| Clade: | Lycophytes |
| Class: | Lycopodiopsida |
| Order: | †Lepidodendrales |
| Families and genera | |
| |
Morphology
Lepidodendrales had tall, thick trunks that rarely branched and were topped with a crown of bifurcating branches bearing clusters of leaves. These leaves were long and narrow, similar to large blades of grass, and were spirally-arranged. The vascular system of the erect trunk was unusual in that it switched its morphological development as the plant grew. The young trunk began as a protostele in which the outer xylem matured first (exarch), but the later and higher portion of the trunk developed as an ectophloic siphonostele in which the xylem was flanked by phloem tissue on both its inner and outer side.[3]
Stem structure
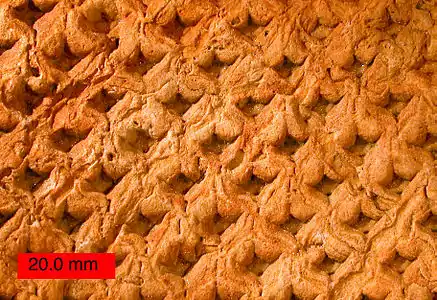
The most common fossil specimens of Lepidodendrales, as well as the most recognizable, are the compressions of stem surfaces marked with constant, though partially asymmetric, rhomboidal leaf cushions. These fossils look much like tire tracks or alligator skin, lending the Greek name "Lepidodendrales," meaning "scale trees." These leaf cushions are actually the expanded leaf base which remained after the leaves fell, as the abscission of the leaf was not flush to the stem surface. The rhomboidal shape arises from the acute angle of the top and bottom of the cushions, known as leaf bolsters, and the rounded angle of the side. The actual leaf scar is present slightly above the midpoint of the cushion and is roughly elliptical in shape. On the leaf scar, three small pitted impressions can sometimes be found. The central and always present pit results from a vascular bundle that extended into the leaf from the stem, known as “parichnos,” a system of aerating tissues. Two other parichnos channels can be found on Lepidodendron stem surfaces, though these do not occur in the Diaphorodendraceae. Above the leaf scar is a mark from a former ligule.[4] A waxy cuticle covered the stem surface, including leaf cushions but not including the stem scars.[5] The simple epidermis lacks specialized cells like trichomes or epidermal glands. Stomata are frequent and sunken in shallow depressions.[6]
Stems of Lepidodendrales could be protostelic, as in Diaphorodendron, have a mixed pith, or be siphonostelic, as in Diaphorodendron and Lepidodendron. In mixed pith stems, parenchyma cells are scattered while tracheids are placed in the middle, though the tracheids exist in a short, squat, parenchymatous shape; this is cited as evidence that the pith in Lepidodendrales originated as immature parenchymatous cells which failed to properly differentiate into tracheids. Around the primary xylem of Lepidodendrales may be a secondary xylem, which can be several centimeters thick. Unlike modern woody trees, the secondary xylem of Lepidodendrales is only a small portion of the diameter of the stem, as the extensively developed periderm is responsible for the large trunks. The primary and secondary xylem tracheids are scalariform and have Williamson striations, or fimbrils, between these scalariform lines. The fimbrils characterize wood in arborescent lycopsids, though similar structures occur in modern club and spike mosses, and these fimbrils are a shared structure for all lycopsids.[7] Bordering outside the secondary xylem is a section of cells with thin walls, representing the vascular cambium. Though modern seed plants have a bifacial cambium, the Lepidodendrids have a unifacial cambium, producing a secondary xylem only on their inner face.[8] The phloem zone is separated from this secondary xylem by a section of thin-walled cells known as the “parenchyma sheath.” Current evidence suggests that there was no secondary phloem present within arborescent lycopsids. The cortex of Lepidodendrids typically consisted of the inner, middle, and outer cortex, distinguished by their cell types. The inner cortex is the narrowest and consists of small parenchyma cells; secretory cells, lacunae, and various sclerotic cells also can be found in this section. The middle cortex is larger and in turn consists of larger parenchyma cells. This section is characterized by radially extending lacunae in young stems, while in older stems the middle cortex is usually not preserved save for a few parenchyma cells. The outer cortex has no definite arrangement, but its cells have slightly thicker walls. The periderm is produced in the outer cortex. The periderm is bizonate in Diaphorodendron, where the inner zone consists of alternatingly thick and thin walled cells, and the outer zone contains dark, “resinous” cells. The homogenous or bizonate periderm is massive in Lepidodendron.[4] The loose construction of the cortex and the large amounts of thin-walled periderm contributed to the sloughing of tissue layers during the fossilization process. This led to a variety of decorticated fossils often presumed to be external stem and trunk features but lacking leaf cushions and other features. Various generic names have been given to decorticated specimens, including Knorria, a name for stems with nearly all tissues outside the xylem absent.[9]
The pattern of stem growth in Lepidodendrales can be reconstructed by analyzing their cortical growth patterns. When plants are immature, the cortex is extensive and the outer stem surface is covered with many rows of leaf bases. As the tree continues to grow, the secondary xylem and periderm originate from the vascular cambium and phellogen. This increase in stem tissue and stem diameter results in the sloughing off of outer tissues including leaf bases; hence, in older areas of the plant the outer surface of the trunk is protected by the periderm. Many older drawings of Lepidodendron incorrectly illustrate leaf bases extending to the ground in older trees. At higher, younger levels of the tree, the branches have fewer rows of smaller leaves. In these sections less secondary xylem and periderm are produced. This reduction in stele size and secondary tissue production continues to taper towards the most distal branches, where only a tiny protostele, no secondary tissues, and few rows of leaves exist; this distal stage of development is known as “apoxogenesis.” These small, distal twigs cannot develop into larger branches over time, a growth pattern known as determinate growth; this contrasts with the modern indeterminate growth pattern of most modern woody plants.[10]
Leaf structure

Leaves of Lepidodendrales plants are linear, with some 1–2 m (3 ft 3 in–6 ft 7 in) long. Stems with the largest diameters have the longest leaves, a pattern correlated with the determinate growth of the plants.[11] Many organ taxa established for detached Lepidodendrales leaves were likely produced by the same kind of plant and differ in morphology only because of their position on the plant. The generic name Lepidophyllum is the original name for preserved Lepidodendrid leaves, but as this name had already been used for a separate flowering plant, the name Lepidophylloides is used today instead.[12] Along the entire lamina of Lepidophylloides, a single vascular bundle is bordered by shallow grooves on the abaxial surface. Stomata are sunken in pits aligned in rows parallel to these grooves. A hypodermal zone of fibers surrounds the vascular bundle of the leaf.[4]
Underground organs

The underground organs of the Lepidodendrales are assigned the generic name Stigmaria. These structures are one of the most common lycopsid fossils and are the main organ found in the clay layer beneath most Carboniferous coal deposits; this clay layer represents the soil layer which the plants were rooted in. Despite the existence of multiple species of Stigmaria, our understanding of the underground organs is based primarily on the widespread species Stigmaria ficoides.[13] The stigmarian organs originate from the base of the trunk as four major axes extending horizontally, leading to a relatively shallow rooting system. Lateral appendages are attached to each axis in a helical pattern. These appendages would abscise as the plant grew, resulting in the characteristic circular external scars of Stigmaria fossil specimens. Although these appendages are often called “stigmarian rootlets,” their helical arrangement and growth abscission are actually more characteristic of leaves than modern lateral roots. The four primary axes of Stigmaria dichotomize often, forming an extensive underground system possibly ranging up to 15 m (49 ft) in radius. The rootlets range in size, being up to 40 cm (16 in) long and 0.5–1 cm (0.20–0.39 in) wide, and typically taper distally and do not dichotomize. A small monarch vascular strand is present in each rootlet, surrounded by a compact inner cortex. Outside this inner cortex is a hollow middle cortex and a thin outer cortex; sometimes a connection extends from the inner cortex to the outer cortex.[14] The primary xylem of Stigmaria is endarch and arranged in a series of bands surrounded by vascular cambium. The secondary xylem tracheids are arranged in radial lines and contain scalariform wall thickenings with fimbrils identical to those in the aerial branches. No secondary phloem has been found in Stigmaria fossil specimens, and the vascular cambium was unifacial with translocation enabled by the primary phloem. The radially aligned tracheids in most Stigmaria axes were produced by a thickening meristem rather than a vascular cambium.[15]
The development of underground organs of Lepidodendrales was likely similar to the development of the aerial stems. However, some features of these organs have yet to be identified in function and some modern features of roots are absent in Stigmaria. The helical arrangement of the rootlet appendages is unlike the irregular arrangement of modern roots. No root hairs have been identified, though fungi in some cortical parenchyma cells may have functioned as mycorrhizae. The monarch vascular bundle in the rootlets is bilaterally symmetrical, but modern roots have radially symmetrical vascular tissue, though vascular bundles in leaves are bilaterally symmetrical. In addition, the rootlets underwent abscission from the axis regularly as the plant grew in a similar fashion to the process of foliar abscission. However, root abscission is unknown in modern plants. These features of the rootlets suggest that they are homologous to the aerial leaves of Lepidodendrales but modified to serve anchoring and absorbing functions. This implies that the underground organs of the plants arose as evolutionary modification of the aerial organs.[16]

Despite the towering 40 m (130 ft) height of some Lepidodendrales plants, their stigmarian system was typically shallow, and therefore it is dubious how the underground organs could support the huge trees, especially since many plants grew in supersaturated, watery soil that was largely unstable. Different suggestions have arisen to explain their stature and root system: it may be that the extensive horizontal growth of the root axes provided enough support, or that the crowns of adjacent trees could entangle and provide mutual support. The nature of the wood and density of the crown of modern trees can have a large effect on tree uprooting, and since arborescent lycopsids had little secondary xylem and bushy crowns they may have been better suited to standing upright.[17]
Reproductive organs

The reproductive organs of the Lepidodendrids consisted of strobili or cones on distal branches in the crown. In Synchysidendron the cones occur on late-formed crown branches, while in Diaphorodendron the cones occur on deciduous lateral branches.[18] The cones could grow to be considerably large, as in Lepidostrobus goldernbergii specimens are over 50 cm (20 in) long. The cones consist of a central axis with sporophylls arranged helically; sporangia are on the adaxial surface of the sporophylls and are upturned distally to overlap sporophylls above. Part of the sporophyll typically extends downward to create a heel or other distal extension. A ligule can be found in a small pit distal to the sporangium. Though Lepidostrubus is the most common name for Lepidodendrales cones, the name has been used for specimens of any form of preservation and for both monosporangiate and bisporangiate forms, so taxonomic problems often ensue.[4] Attempts to dissuade these taxonomic confusions have been made. Some have suggested the name Lepidostrobus should only describe monosporangiate cones and the name Flemingites describe bisporangiate cones, while others have used cone morphology to attempt to better differentiate species within Lepidostrobus.[19][20]
Embryo specimens have been found in the cone Bothrodendrostrobus.[21] The embryo begins as an unvascularized globular structure found within megagametophyte tissue, and in more mature specimens two vascularized appendages extend through the trilete suture, representing the first shoot and first root. Gametophyte generation of Lepidodendrales is poorly understood and based on few specimens, but the Flemingites schopfii cones exhibit well-preserved signs of the micro and megametophyte phases.[22] Compared to gametophytes of modern lycopsids, F. schopfii has microgametophytes most similar to extant Selaginella, while the megagametophytes are more similar to Isoetes. Other well-preserved Lepidodendrid gametophytes have been found in spores of Lepidodendron rhodumnense fossilized in chert from the late Viséan.[23]
Ecology
The large quantities of biomass that were responsible for the formation of globally widespread Carboniferous coal seams can be traced to members of the Lepidodendrales group; Lepidodendrales members can be traced back to almost 70% of the plant material in the Westphalian coal-swamp forests of America,[24] though at the end of the Westphalian period Lepidodendrales members were in decline and had become responsible for only 5% of coal biomass.[25] Arborescent lycopsids were largely becoming extinct in North America and Europe by the end of the Carboniferous as tree ferns began to rise to prominence, though arborescent lycopsids persisted in China until the Middle Permian.[4] Some scientists have suggested that the decline of lepidodendrids during this period was a result of Variscan tectonic activity creating unstable conditions by reducing the size of the coal-swamp ecosystems,[25] while others suggest that their decline was due to climate change; some scientists suggest a combination of these theories, that tectonic activity caused changes in floral composition which triggered climate change, in turn resulting in this decline.[26]
Genera
Various specimens of Lepidodendrales have been historically categorized as members of Lepidodendron, a genus defined by morphology of leaf cushions. DiMichele established Diaphorodendron to dissuade ambiguity over these widely ranging specimens, which includes some structurally preserved specimens which were previously members of Lepidodendron. Diaphorodendron was later divided into the two genera Diaphorodendron and Synchysidendron, and the genera were placed in the new family Diaphorodendraceae.[18] Synamorphies of this new family are a medullated protostele and a dorsiventrally flattened megasporangium. Synamorphies of the family Lepidodendraceae are a bilaterally flattened megasporangium and infrafoliar parichnos which extend below the leaf scar.[27] The generic names Lepidodendron and Diaphorodendron today describe both cellularly preserved stem segments and entire plants, including their foliar organs, underground organs, and reproductive organs. Specifically, the generic name Lepidodendron is typically used to describe compression specimens which feature a particular type of leaf cushion morphology.[4]
In addition, many "organ taxa" have been identified to the Lepidodendrales: roots (Stigmaria), leaves, and cones (Lepidostrobus) were originally given a different genus and species name before it could be shown that they belonged to the same organism.
See also
References
- A. V. Lopatin (2012). Палеонтологический музей имени Ю.А. Орлова (The Orlov Museum of Paleontology). Moscow: PIN RAN. p. 56. ISBN 978-5-903825-14-1. Retrieved 2020-10-05.
- V. V. Alekhin (1961). Geografiia rastenii s osnovani botaniki (Geography of plants and basics of botany). Gos. nauchno-pedagog. izd-vo. p. 167. Retrieved 2020-10-05.
- Bold, Harold C.; C. J. Alexopoulos; T. Delevoryas (1987). Morphology of Plants and Fungi (5th ed.). New York: Harper-Collins. pp. 496–503. ISBN 0-06-040839-1.
- Taylor, Edith L.; Taylor, Thomas N.; Krings, Michael (2009). Paleobotany: the biology and evolution of fossil plants. Academic Press.
- Thomas, B. A. (1966). "The cuticle of the lepidodendroid stem". New Phytologist. Wiley Online Library. 65 (3): 296–303. doi:10.1111/j.1469-8137.1966.tb06365.x.
- Thomas, B. A. (1974). "THE LEPIDODENDROID STOMA". Cite journal requires
|journal=(help) - Wilder, George J. (1970). "Structure of tracheids in three species of Lycopodium". American Journal of Botany. Wiley Online Library. 57 (9): 1093–1107. doi:10.1002/j.1537-2197.1970.tb09913.x.
- Eggert, D. A.; Kanemoto, N. Y. (1977). "Stem phloem of a middle Pennsylvanian Lepidodendron". Botanical Gazette. The University of Chicago Press. 138 (1): 102–111. doi:10.1086/336903.
- Hirmer, Max; Pia, Julius; Troll, Wilhelm (1927). "Handbuch der Palaobotanik: Thallophyta-Bryophyta-Pteridophyta". 1. R. Oldenbourg. Cite journal requires
|journal=(help) - Eggert, Donald Alan (1961). "The ontogeny of Carboniferous arborescent Lycopsida". Palaeontographica Abteilung. Schweizerbart'sche Verlagsbuchhandlung: 43–92.
- Chaloner, W. G.; Meyer-Berthaud, B. (1983). "Leaf and stem growth in the Lepidodendrales". Botanical Journal of the Linnean Society. Oxford University Press. 86 (1–2): 135–148. doi:10.1111/j.1095-8339.1983.tb00721.x.
- Rychnovský, Vojtěch (2013). "Revize druhů Lepidodendron lycopodioides a L. selaginoides z českého karbonu". Univerzita Karlova, Přírodovědecká fakulta. Cite journal requires
|journal=(help) - Williamson, William Crawford (1887). A monograph on the morphology and histology of Stigmaria ficoides. 40. Palaeontographical society.
- Weiss, Frederick Ernest (1902). "The Vascular branches of Stigmarian rootlets". Annals of Botany. JSTOR. 16 (63): 559–573. JSTOR 43235190.
- Rothwell, Gar W.; Pryor, Janelle S. (1991). "Developmental dynamics of arborescent lycophytes—apical and lateral growth in Stigmaria ficoides". American Journal of Botany. iley Online Library. 78 (12): 1740–1745. doi:10.1002/j.1537-2197.1991.tb14538.x.
- Frankenberg, Julian M.; Eggert, Donald A. (1969). "Petrified Stigmaria from North America: Part I. Stigmaria ficoides, the unterground portions of Lepidodendraceae". Palaeontographica Abteilung. Schweizerbart'sche Verlagsbuchhandlung: 1–47.
- Niklas, Karl J. (1992). Plant biomechanics: an engineering approach to plant form and function. University of Chicago press. ISBN 9780226586311.
- DiMichele, William A.; Bateman, Richard M. (1992). "Diaphorodendraceae, fam. nov.(Lycopsida: Carboniferous): systematics and evolutionary relationships of Diaphorodendron and Synchysidendron, gen. nov". American Journal of Botany. Wiley Online Library. 79 (6): 605–617. doi:10.1002/j.1537-2197.1992.tb14602.x.
- Brack-Hanes, Sheila D.; Thomas, Barry A. (1983). "A re-examination of Lepidostrobus Brongniart". Botanical Journal of the Linnean Society. Oxford University Press. 86 (1–2): 125–133. doi:10.1111/j.1095-8339.1983.tb00720.x.
- Bek, Jiří; Opluštil, Stanislav (2006). "Six rare Lepidostrobus species from the Pennsylvanian of the Czech Republic and their bearing on the classification of lycospores". Review of Palaeobotany and Palynology. Elsevier. 139 (1–4): 211–226. doi:10.1016/j.revpalbo.2006.01.003.
- Stubblefield, Sara P.; Rothwell, Gar W. (1981). "Embryogeny and reproductive biology of Bothrodendrostrobus mundus (Lycopsida)". American Journal of Botany. Wiley Online Library. 68 (5): 625–634. doi:10.1002/j.1537-2197.1981.tb12394.x.
- Brack-Hanes, Sheila D. (1978). "On the megagametophytes of two lepidodendracean cones". Botanical Gazette. The University of Chicago Press. 139 (1): 140–146. doi:10.1086/336979.
- Galtier, J. (1964). "PALEOBOTANIQUE-SUR LE GAMETOPHYTE FEMELLE DES LEPIDODENDRACEES". Comptes Rendus Hebdomadaires des Séances de l'Académie des Sciences. GAUTHIER-VILLARS/EDITIONS ELSEVIER 23 RUE LINOIS, 75015 PARIS, FRANCE. 258 (9): 2625.
- DiMichele, William A. (1985). "Diaphorodendron, gen. nov., a segregate from Lepidodendron (Pennsylvanian age)". Systematic Botany. JSTOR. 10 (4): 453–458. doi:10.2307/2419138. JSTOR 2419138.
- Kerp, Hans (2000). "The modernization of landscapes during the Late Paleozoic-Early Mesozoic". The Paleontological Society Papers. Cambridge University Press. 6: 79–114. doi:10.1017/S1089332600000723.
- DiMichele, William A.; Phillips, Tom L. (1996). "Climate change, plant extinctions and vegetational recovery during the Middle-Late Pennsylvanian transition: The case of tropical peat-forming environments in North America". Geological Society, London, Special Publications. Geological Society of London. 102 (1): 201–221. doi:10.1111/j.1472-4669.2005.00043.x.
- DiMichele, William A.; Bateman, Richard M. (1996). "The rhizomorphic lycopsids: a case-study in paleobotanical classification". Systematic Botany. JSTOR. 21 (4): 535–552. doi:10.2307/2419613. JSTOR 2419613.
Further reading
- Davis, Paul and Kenrick, Paul. Fossil Plants. Smithsonian Books, Washington D.C. (2004).
- Morran, Robin, C.; A Natural History of Ferns. Timber Press (2004). ISBN 0-88192-667-1
