Lutetium phthalocyanine
Lutetium phthalocyanine (LuPc
2) is a coordination compound derived from lutetium and two phthalocyanines. It was the first known example of a molecule that is an intrinsic semiconductor.[1][2] It exhibits electrochromism, changing color when subject to a voltage.
| |||
| |||
| Names | |||
|---|---|---|---|
| Other names
Lutetium bisphthalocyanine Lutetium biphthalocyanine | |||
| Identifiers | |||
3D model (JSmol) |
|||
| |||
| |||
| Properties | |||
| LuC 64H 32N 16 | |||
| Molar mass | 1200.04 g/mol | ||
| Appearance | green solid; red when oxidized; blue when reduced | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
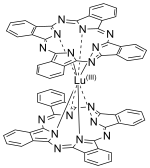
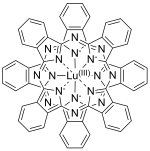
Structure
LuPc
2 is a double-decker sandwich compound consisting of a Lu3+ ion coordinated to two the conjugate base of two phthalocyanines. The rings are arranged in a staggered conformation. The extremities of the two ligands are slightly distorted outwards.[3] The complex features a non-innocent ligand, in the sense that the macrocycles carry an extra electron.[4] It is a free radical[1] with the unpaired electron sitting in a half-filled molecular orbital between the highest occupied and lowest unoccupied orbitals, allowing its electronic properties to be finely tuned.[3]
Properties
LuPc
2, along with many substituted derivatives like the alkoxy-methyl derivative Lu[(C
8H
17OCH
2)
8Pc]
2, can be deposited as a thin film with intrinsic semiconductor properties;[4] said properties arise due to its radical nature[1] and its low reduction potential compared to other metal phthalocyanines.[2] This initially green film exhibits electrochromism; the oxidized form LuPc+
2 is red, whereas the reduced form LuPc−
2 is blue and the next two reduced forms are dark blue and violet, respectively.[4] The green/red oxidation cycle can be repeated over 10,000 times in aqueous solution with dissolved alkali metal halides, before it is degraded by hydroxide ions; the green/blue redox degrades faster in water.[4]
Electrical properties
LuPc
2 and other lanthanide phthalocyanines are of interest in the development of organic thin-film field-effect transistors.[3][5]
LuPc
2 derivatives can be selected to change color in the presence of certain molecules, such as in gas detectors;[2] for example, the thioether derivative Lu[(C
6H
13S)
8Pc]
2 changes from green to brownish-purple in the presence of NADH.[6]
References
- Belarbi, Z.; Sirlin, C.; Simon, J.; Andre, Jean Jacques (November 1989). "Electrical and magnetic properties of liquid crystalline molecular materials: lithium and lutetium phthalocyanine derivatives". The Journal of Physical Chemistry. 93 (24): 8105–8110. doi:10.1021/j100361a026.
- Trometer, M.; Even, R.; Simon, J.; Dubon, A.; Laval, J.-Y.; Germain, J.P.; Maleysson, C.; Pauly, A.; Robert, H. (May 1992). "Lutetium bisphthalocyanine thin films for gas detection". Sensors and Actuators B: Chemical. 8 (2): 129–135. doi:10.1016/0925-4005(92)80169-X.
- Bidermane, I.; Lüder, J.; Boudet, S.; Zhang, T.; Ahmadi, S.; Grazioli, C.; Bouvet, M.; Rusz, J.; Sanyal, B.; Eriksson, O.; Brena, B.; Puglia, C.; Witkowski, N. (21 June 2013). "Experimental and theoretical study of electronic structure of lutetium bi-phthalocyanine". The Journal of Chemical Physics. 138 (23): 234701. doi:10.1063/1.4809725. ISSN 0021-9606.
- Toupance, Thierry; Plichon, Vincent; Simon, Jacques (1999). "Substituted bis(phthalocyanines): electrochemical properties and probe beam deflection (mirage) studies". New Journal of Chemistry. 23 (10): 1001–1006. doi:10.1039/A905248H.
- Wang, Jun; Wang, Haibo; Zhang, Jian; Yan, Xuanjun; Yan, Donghang (15 January 2005). "Organic thin-film transistors with improved characteristics using lutetium bisphthalocyanine as a buffer layer". Journal of Applied Physics. 97 (2): 026106. doi:10.1063/1.1840093.
- Basova, Tamara; Gürek, Ayşe Gül; Ahsen, Vefa; Ray, Asim (1 January 2013). "Electrochromic lutetium phthalocyanine films for in situ detection of NADH". Optical Materials. 35 (3): 634–637. doi:10.1016/j.optmat.2012.10.017.