Malononitrile
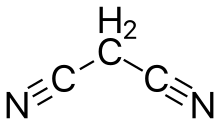
Malononitrile, also propanedinitrile or malonodinitrile, is a nitrile with the formula CH2(CN)2.
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Malononitrile[1] | |||
| Systematic IUPAC name
Propanedinitrile[2] | |||
| Other names
Cyanoacetonitrile, Dicyanomethane, Malonic dinitrile[3] | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 773697 | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.368 | ||
| EC Number |
| ||
| 1303 | |||
| MeSH | dicyanmethane | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2647 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C3H2N2 | |||
| Molar mass | 66.063 g·mol−1 | ||
| Appearance | Colourless crystals or white powder[3] | ||
| Density | 1.049 g mL−1 | ||
| Melting point | 32 °C; 89 °F; 305 K | ||
| Boiling point | 220.1 °C; 428.1 °F; 493.2 K | ||
| 13% (20 °C)[3] | |||
| Thermochemistry | |||
Heat capacity (C) |
110.29 J K−1 mol−1 | ||
Std molar entropy (S |
130.96 J K−1 mol−1 | ||
Std enthalpy of formation (ΔfH⦵298) |
187.7–188.1 kJ mol−1 | ||
Std enthalpy of combustion (ΔcH⦵298) |
−1.6540–−1.6544 MJ mol−1 | ||
| Hazards | |||
| GHS pictograms |  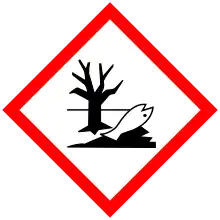 | ||
| GHS Signal word | Danger | ||
| H301, H311, H331, H410 | |||
| P261, P273, P280, P301+310, P311 | |||
| Flash point | 86 °C (187 °F; 359 K) | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
| ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
none[3] | ||
REL (Recommended) |
TWA 3 ppm (8 mg/m3)[3] | ||
IDLH (Immediate danger) |
N.D.[3] | ||
| Related compounds | |||
Related alkanenitriles |
|||
Related compounds |
DBNPA | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Malononitrile is relatively acidic, with a pKa of 11 in water.[4] This allows it to be used in the Knoevenagel condensation, for example in the preparation of CS gas:
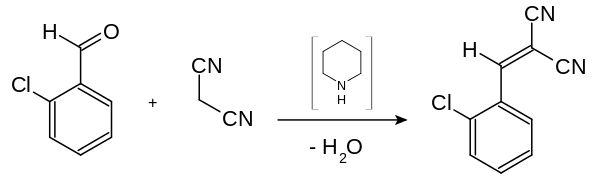
CS-chemical-synthesis
In related chemistry, malononitrile is a suitable starting material for the Gewald reaction, where the nitrile condenses with a ketone or aldehyde in the presence of elemental sulfur and a base to produce a 2-aminothiophene.[5]
See also
References
- ChemSpider lists the name 'malononitrile' as a valid, expert-verified IUPAC name.
- "dicyanmethane - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification. Retrieved 7 June 2012.
- NIOSH Pocket Guide to Chemical Hazards. "#0378". National Institute for Occupational Safety and Health (NIOSH).
- Evans pKa table
- Sabnis, R.W.; Rangnekar, D.W.; Sonawane, N.D. (1999). "2-Aminothiophenes By The Gewald Reaction". Journal of Heterocyclic Chemistry. 36 (2): 333–345. doi:10.1002/jhet.5570360203. Retrieved 2007-07-18.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.


