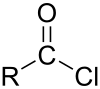
Malonyl chloride
Malonyl chloride is the organic compound with the formula CH2(COCl)2. It is the acyl chloride derivative of malonic acid and the simplest three-carbon diacid chloride. It is a colorless liquid although samples are often deeply colored owing to impurities. The compound degrades at room temperature after a few days. It used as a reagent in organic synthesis.[1]
2.png.webp) | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.015.249 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H2Cl2O2 | |
| Molar mass | 140.95 g·mol−1 |
| Appearance | colorless liquid |
| Boiling point | 58 °C (136 °F; 331 K) 28 mm Hg |
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Danger |
| H226, H314 | |
| P210, P233, P240, P241, P242, P243, P260, P264, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P363, P370+378, P403+235, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis and reactions
Malonyl chloride can be synthesized from malonic acid in thionyl chloride.[2] As a bifunctional compound, it is used in the preparation of a number of cyclic compounds by diacylation. Heating in the presence of non-nucleophilic base gives the ketene derivative O=C=C(H)COCl.
References
- Thomas Ziegler (2001). "Malonyl Chloride". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rm016.
- Chittaranjan Raha (1953). "Di-tert-Butyl Malonate". Organic Syntheses. 33: 20. doi:10.15227/orgsyn.034.0026.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.