Organomolybdenum chemistry
Organomolybdenum chemistry is the chemistry of chemical compounds with Mo-C bonds. The heavier group 6 elements molybdenum and tungsten form organometallic compounds similar to those in organochromium chemistry but higher oxidation states tend to be more common.[2]
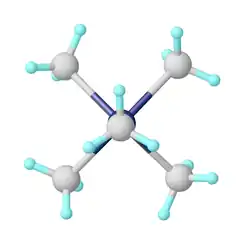
Mo(0) and more reduced states
Molybdenum hexacarbonyl is the precursor to many substituted derivatives. It reacts with organolithium reagents to give anionic acyls which can be O-alkylated to give Fischer carbenes.
molybdenum_tricarbonyl.png.webp)
Mo(CO)6 reacts with arenes to give piano-stool complexes such as (mesitylene)molybdenum tricarbonyl. Cycloheptatrienemolybdenum tricarbonyl, which is related to (arene)Mo(CO)3, reacts with trityl salts to give the cycloheptatrienyl complex:[3]
- (C7H8)Mo(CO)3 + (C6H5)3C+ → [(C7H7)Mo(CO)3]+ + (C6H5)3CH
3.png.webp)
Reduction of Mo(CO)6 gives [Mo(CO)5]2− which is formally Mo(-II).[4]
CO-free Mo(0) compounds tend to be more reducing and kinetically labile than the carbonyl complexes.[5] Examples include bis(benzene)molybdenum (Mo(C6H6)2) and tris(butadiene)molybdenum. Such compounds can be prepared by metal vapor synthesis and reductive routes from molybdenum(V) chloride.[6]
Mo(II)
Halogenation of Mo(CO)6 gives Mo(II) carbonyl halides, which are also versatile precursors.[7] One large collection of compounds have the formula (C5R5)Mo(CO)3X, derived from cyclopentadienylmolybdenum tricarbonyl dimer (X = halide, hydride, alkyl).[8]
Treating molybdenum(II) acetate with methyllithium gives Li4[Mo2(CH3)8].
Mo(IV)
With the formula of the type Cp2MoX2 molybdocene dichloride (X = Cl) and molybdocene dihydride (X = H) are both known as are ansa metallocene analogues.
Mo(V) and Mo(VI)
Mo(CH3)5, Mo(CH3)6, and salts of [Mo(CH3)7]- are known.[5]
Oxo and imide (RN=) ligands are found in several high oxidation state organomolybdenum compounds. The complexes (C5R5)MoO2X are illustrative.[9] Schrock's Mo-based olefin metathesis catalysts feature molybdenum(VI) centers supported by alkoxide, alkylidene, and imido ligands.[10]
Organotungsten compounds
Tungsten analogues of almost all organoMo compounds are known. Some notable examples include hexamethyltungsten and analogues of Schrock olefin metathesis catalysts.
Applications
Mo-based catalysts are useful for olefin metathesis.[10]

Trisamidomolybdenum(VI) alkylidyne complexes catalyze alkyne metathesis.[11]
In the Kauffmann olefination, molybdenum(III) chloride and methyllithium form an organometallic complex capable of carbonyl olefination.[12]
References
- Beatrice Roessler; Sven Kleinhenza; Konrad Seppelt (2000). "Pentamethylmolybdenum". Chemical Communications (12): 1039–1040. doi:10.1039/b000987n.
- Poli, R. (2008). "High oxidation state organomolybdenum and organotungsten chemistry in protic environments". Coord. Chem. Rev. 252 (15–17): 1592–1612. doi:10.1016/j.ccr.2007.11.029.
- Green M. L. H., Ng D. K. P. (1995). "Cycloheptatriene and -enyl Complexes of the Early Transition Metals". Chemical Reviews. 95: 439–73. doi:10.1021/cr00034a006.
- Ellis, J. E. (2003). "Metal Carbonyl Anions: from [Fe(CO)4]2− to [Hf(CO)6]2− and Beyond". Organometallics. 22 (17): 3322–3338. doi:10.1021/om030105l.
- Flower, K. R. (2007). "Molybdenum compounds without CO or isonitrile ligands". In Mingos, D. Michael P.; Crabtree, Robert H. (eds.). Comprehensive Organometallic Chemistry III. 5. pp. 513–595. doi:10.1016/B0-08-045047-4/00072-8. ISBN 9780080450476.
- Stephan, G. C.; Naether, C.; Peters, G.; Tuczek, F. (2013). "Molybdenum 17- and 18-Electron Bis- and Tris(Butadiene) Complexes: Electronic Structures, Spectroscopic Properties, and Oxidative Ligand Substitution Reactions". Inorg. Chem. 52 (10): 5931–5942. doi:10.1021/ic400145f. PMID 23627292.
- Joseph L. Templeton "Four-Electron Alkyne Ligands in Molybdenum(II) and Tungsten(II) Complexes" Advances in Organometallic Chemistry 1989, Volume 29, Pages 1–100.doi:10.1016/S0065-3055(08)60352-4
- Synthesis of Organometallic Compounds: A Practical Guide Sanshiro Komiya Ed. S. Komiya, M. Hurano 1997
- Kuehn, F. E.; Santos, A. M.; Herrmann, W. A. (2005). "Organorhenium(VII) and Organomolybdenum(VI) Oxides: Syntheses and Application in Olefin Epoxidation". Dalton Trans. (15): 2483–2491. doi:10.1039/b504523a. PMID 16025165.
- R.R. Schrock (1986). "High-oxidation-state molybdenum and tungsten alkylidene complexes". Acc. Chem. Res. 19 (11): 342–348. doi:10.1021/ar00131a003.
- Wei Zhang; Yunyi Lu; Jeffrey S. Moore (2007). "Preparation of a Trisamidomolybdenum(VI) Propylidyne Complex". Org. Synth. 84: 163. doi:10.15227/orgsyn.084.0163.Wei Zhang; Hyeon Mo Cho; Jeffrey S. Moore (2007). "Preparation of a Carbazole-Based Macrocycle via Precipitation-driven Alkyne Metathesis". Org. Synth. 84: 177. doi:10.15227/orgsyn.084.0177. S2CID 93992722.
- Kauffmann, T. (1997). "Organomolybdenum and organotungsten reagents. 7. Novel reactions of organomolybdenum and organotungsten compounds: additive-reductive carbonyl dimerization, spontaneous transformation of methyl ligands into μ-methylene ligands, and selective carbonylmethylenation". Angew. Chem. Int. Ed. Engl. 36: 1259–1275. doi:10.1002/anie.199712581.