Monolignol
Monolignols, also called lignols, are the source materials for biosynthesis of both lignans and lignin.[1] The starting material for production of monolignols is the amino acid phenylalanine. Via the phenylpropanoid pathway, phenylalanine is first converted to paracoumaryl alcohol. Paracoumaryl alcohol is subsequently elaborated to coniferyl alcohol and sinapyl alcohol.
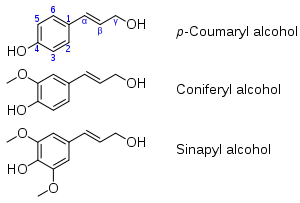
These three alcohols - paracoumaryl alcohol, coniferyl alcohol, and sinapyl alcohol - are key phytochemicals in the production of lignans and lignin. The conversion entails oxidative coupling reactions, which usually involve the propenyl substituents. Lignans are typically dimers of lignols and lignin is a polymer. Lignans are soluble and thus susceptible to biodegradation, whereas lignin is inert, as appropriate for the material that forms the structures of woody plants.
The ratio of the three lignols varies with plant species. For example, Norway spruce lignin is almost entirely coniferyl alcohol, whereas paracoumaryl alcohol is found almost exclusively in grasses.
Monolignols are biosynthetised in the cytosol and linked to glucose, i.e. they are converted to glucosides. The glucosyl group confers water-solublity. The glucosides are transported through the cell membrane to the apoplast. The glucose is then removed and the monolignols are polymerised into lignin.
The phenylpropenes are derived from the monolignols.
References
- W. Boerjan; J. Ralph; M. Baucher (June 2003). "Lignin biosynthesis". Annu. Rev. Plant Biol. 54 (1): 519–549. doi:10.1146/annurev.arplant.54.031902.134938. PMID 14503002.