Myrosinase
Myrosinase (EC 3.2.1.147, thioglucoside glucohydrolase, sinigrinase, and sinigrase) is a family of enzymes involved in plant defense against herbivores, specifically the mustard oil bomb. The three-dimensional structure has been elucidated and is available in the PDB (see links in the infobox).
| Thioglucosidase (Myrosinase) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| EC number | 3.2.1.147 | ||||||||
| CAS number | 9025-38-1 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
A member of the glycoside hydrolase family, myrosinase possesses several similarities with the more ubiquitous O-glycosidases.[2][3] However, myrosinase is the only known enzyme found in nature that can cleave a thio-linked glucose. Its known biological function is to catalyze the hydrolysis of a class of compounds called glucosinolates.[4]
Myrosinase activity
Myrosinase is regarded as a defense-related enzyme and is capable of hydrolyzing glucosinolates into various compounds, some of which are toxic.[5]
Mechanism
Myrosinase catalyzes the chemical reaction
- a thioglucoside + H2O a sugar + a thiol
Thus, the two substrates of this enzyme are thioglucoside and H2O, whereas its two products are sugar and thiol.
In the presence of water, myrosinase cleaves off the glucose group from a glucosinolate. The remaining molecule then quickly converts to a thiocyanate, an isothiocyanate, or a nitrile; these are the active substances that serve as defense for the plant. The hydrolysis of glucosinolates by myrosinase can yield a variety of products, depending on various physiological conditions such as pH and the presence of certain cofactors. All known reactions have been observed to share the same initial steps. (See Figure 2.) First, the β-thioglucoside linkage is cleaved by myrosinase, releasing D-glucose. The resulting aglycone undergoes a spontaneous Lossen-like rearrangement, releasing a sulfate. The last step in the mechanism is subject to the greatest variety depending on the physiological conditions under which the reaction takes place. At neutral pH, the primary product is the isothiocyanate. Under acidic conditions (pH < 3), and in the presence of ferrous ions or epithiospecifer proteins, the formation of nitriles is favored instead.[2][6]
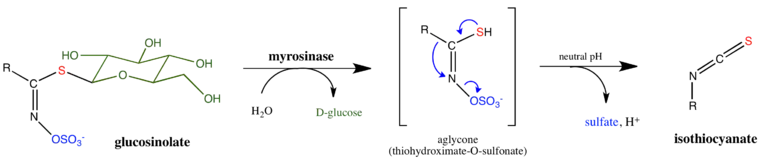
Cofactors and inhibitors
Ascorbate is a known cofactor of myrosinase, serving as a base catalyst in glucosinolate hydrolysis.[1][7] For example, myrosinase isolated from daikon (Raphanus sativus) demonstrated an increase in V max from 2.06 μmol/min per mg of protein to 280 μmol/min per mg of protein on the substrate, allyl glucosinolate (sinigrin) when in the presence of 500 μM ascorbate.[4] Sulfate, a byproduct of glucosinolate hydrolysis, has been identified as a competitive inhibitor of myrosinase.[4] In addition, 2-F-2-deoxybenzylglucosinolate, which was synthesized specifically to study the mechanism of myrosinase, inhibits the enzyme by trapping one of the glutamic acid residues in the active site, Glu 409.[3][8]
Structure
Myrosinase exists as a dimer with subunits of 60-70 kDa each.[9] [10] X-ray crystallography of myrosinase isolated from Sinapis alba revealed the two subunits are linked by a zinc atom.[7] The prominence of salt bridges, disulfide bridges, hydrogen bonding, and glycosylation are thought to contribute to the enzyme’s stability, especially when the plant is under attack and experiences severe tissue damage.[2] A feature of many β-glucosidases are catalytic glutamate residues at their active sites, but two of these have been replaced by a single glutamine residue in myrosinase.[3][11] Ascorbate has been shown to substitute for the activity of the glutamate residues.[1] (See Figure 3 for mechanism.)
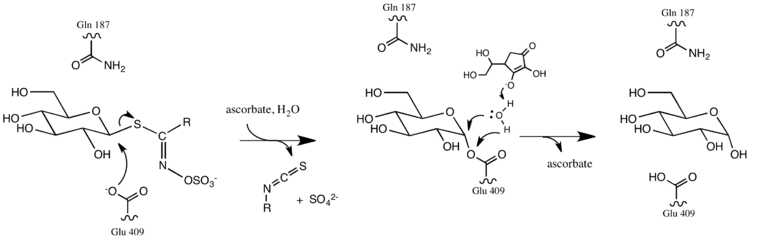
Biological function
Myrosinase and its natural substrate, glucosinolate, are known to be part of the plant’s defense response. When the plant is attacked by pathogens, insects, or other herbivores, the plant uses myrosinase to convert glucosinolates, which are otherwise-benign, into toxic products like isothiocyanates, thiocyanates, and nitriles.[2]
Compartmentalization in plants
The glucosinolate-myrosinase defensive system is packaged in the plant in a unique manner. Plants store myrosinase glucosinolates by compartmentalization, such that the latter is released and activated only when the plant is under attack. Myrosinase is stored largely as myrosin grains in the vacuoles of particular idioblasts called myrosin cells, but have also been reported in protein bodies or vacuoles, and as cytosolic enzymes that tend to bind to membranes.[12][13] Glucosinolates are stored in adjacent but separate "S-cells." [14] When the plant experiences tissue damage, the myrosinase comes into contact with glucosinolates, quickly activating them into their potent, antibacterial form.[2] The most potent of such products are isothiocyanates, followed by thiocyanates and nitriles.[15]
Evolution
Plants known to have evolved a myrosinase-glucosinolate defense system include: white mustard (Sinapis alba), [9] garden cress (Lepidium sativum),[16] wasabi (Wasabia japonica),[17] daikon (Raphanus sativus),[18][19] as well as several members of the family Brassicaceae, including yellow mustard (Brassica juncea),[20] rape seed (Brassica napus),[21] and common dietary brassicas like broccoli, cauliflower, cabbage, bok choy, and kale. [2] The bitter aftertaste of many of these vegetables can often be attributed to the hydrolysis of glucosinolates upon tissue damage during food preparation or when consuming these vegetables raw.[2] Papaya seeds use this method of defense, but not the fruit pulp itself.[22]
Myrosinase has also been isolated from the cabbage aphid.[23] This suggests coevolution of the cabbage aphid with its main food source. The aphid employs a similar defense strategy to plants. Like its main food source, the cabbage aphid compartmentalizes its native myrosinase and the glucosinolates it ingests. When the cabbage aphid is attacked and its tissues are damaged, its stored glucosinolates are activated, producing isothiocyanates and deterring predators from attacking other aphids.[24]
Historical relevance and modern applications
Agriculture
Historically, crops like rapeseed that contained the glucosinolate-myrosinase system were deliberately bred to minimize glucosinolate content, since rapeseed in animal feed was proving toxic to livestock.[25] The glucosinolate-myrosinase system has been investigated as a possible biofumigant to protect crops against pests. The potent glucosinolate hydrolysis products (GHPs) could be sprayed onto crops to deter herbivory. Another option would be to use techniques in genetic engineering to introduce the glucosinolate-myrosinase system in crops as a means of fortifying their resistance against pests.[15]
Human health
Isothiocyanates, the primary product of glucosinolate hydrolysis, have been known to prevent iodine uptake in the thyroid, causing goiters.[26] Isothiocyanates in high concentrations have also been known to cause hepatotoxicity, or liver damage.[4] However, more recent studies have shown that diets high in glucosinolate-containing vegetables such as dietary brassicas have been associated with lower risks of heart disease, diabetes, and cancer.[2][27] Isothiocyanates have been shown to induce phase II detoxification enzymes involved in the xenobiotic metabolism of carcinogens.[28] There has been increasing evidence to suggest that a myrosinase-like enzyme may also be present in members of the human gut microbiome. Although myrosinase, like many enzymes, will be denatured at high temperatures and thus lose its activity when cooked, a gut microbe capable of catalyzing the same hydrolysis of glucosinolates would be able to activate ingested glucosinolates into their more potent forms, e.g. isothiocyanates.[29][30]
According to an article in The New England Journal of Medicine, a Chinese woman who ate 1–1.5 kg (2.2–3.3 lb) of raw bok choy daily developed severe hypothyroidism due to excessive ingestion of myrosinase.[31]
References
- Burmeister, W. P.; Cottaz, S.; Rollin, P.; Vasella, A.; Henrissat, B. (2000). "High Resolution X-ray Crystallography Shows That Ascorbate is a Cofactor for Myrosinase and Substitutes for the Function of the Catalytic Base". Journal of Biological Chemistry. 275 (50): 39385–39393. doi:10.1074/jbc.M006796200. PMID 10978344.
- Halkier, B. A.; Gershenzon, J. (2006). "Biology and Biochemistry of Glucosinolates". Annual Review of Plant Biology. 57: 303–333. doi:10.1146/annurev.arplant.57.032905.105228. PMID 16669764.
- Bones, A. M.; Rossiter, J. T. (2006). "The enzymic and chemically induced decomposition of glucosinolates". Phytochemistry. 67 (11): 1053–1067. doi:10.1016/j.phytochem.2006.02.024. PMID 16624350.
- Shikita, M.; Fahey, J. W.; Golden, T. R.; Holtzclaw, D.; and Talalay, P. (2000). "An unusual case of "uncompetitive activation" by ascorbic acid: Purification and kinetic properties of a myrosinase from Raphanus sativus seedlings". Journal of Biochemistry. 341 (3): 725–732. doi:10.1042/0264-6021:3410725. PMC 1220411. PMID 10417337.
- A wound- and methyl jasmonate-inducible transcript coding for a myrosinase-associated protein with similarities to an early nodulin
- Lambrix, V.; et al. (2001). "The Arabidopsis Epithiospecifier Protein Promotes the Hydrolysis of Glucosinolates to Nitriles and Influences Trichoplusia ni Herbivory". The Plant Cell. 13 (12): 2793–2807. doi:10.1105/tpc.010261. PMC 139489. PMID 11752388.
- Burmeister, W. P.; Cottaz, S.; Driguez, H.; Iori, R.; Palmieri, S.; Henrissat (1997). "The crystal structures of Sinapis alba myrosinase and a covalent glycosyl–enzyme intermediate provide insights into the substrate recognition and active-site machinery of an S-glycosidase". Structure. 5 (5): 663–675. doi:10.1016/s0969-2126(97)00221-9. PMID 9195886.
- Cottaz, S.; Rollin, P.; Driguez, H. (1997). "Synthesis of 2-deoxy-2-fluoroglucotropaeolin, a thioglucosidase inhibitor". Carbohydrate Research. 298 (1–2): 127–130. doi:10.1016/s0008-6215(96)00294-7.
- Björkman, R.; Janson, J.-C. (1972). "Studies on myrosinases". Biochim. Biophys. Acta. 276 (2): 508–518. doi:10.1016/0005-2744(72)91011-X.
- Pessina, A.; Thomas, R. M.; Palmieri, S.; Luisi, P. L. (1990). "An improved method for the purification of myrosinase and its physicochemical characterization". Arch. Biochem. Biophys. 280 (2): 383–389. doi:10.1016/0003-9861(90)90346-Z.
- Henrissat, B.; Davies, J. G. (2000). "Glycoside Hydrolases and Glycosyltransferases: Families, Modules, and Implications for Genomics". Plant Physiology. 124 (4): 1515–1519. doi:10.1104/pp.124.4.1515. PMC 1539306. PMID 11115868.
- Luthy, B; Matile, P (1984). "The mustard oil bomb - rectified analysis of the subcellular organization of the myrosinase system". Biochem Physiol PFL. 179 (1–2): 5–12. doi:10.1016/s0015-3796(84)80059-1.
- Andréasson, E. (2001). "Different Myrosinase and Idioblast Distribution in Arabidopsis and Brassica napus". Plant Physiology. 127 (4): 1750–1763. doi:10.1104/pp.010334. PMC 133578. PMID 11743118.
- Koroleva, O. A.; et al. (2000). "Identification of a new glucosinolate-rich cell type in Arabidopsis flower stalk". Plant Physiol. 124 (2): 599–608. doi:10.1104/pp.124.2.599. PMC 59166. PMID 11027710.
- Gimsing, A. L.; Kirkegaard, J. A. (2009). "Glucosinolates and biofumigation: fate of glucosinolates and their hydrolysis products in soil". Phytochem Rev. 8: 299–310. doi:10.1007/s11101-008-9105-5.
- Durham, P.; Poulton, J. E. (1989). "Effect of Castanospermine and Related Polyhydroxyalkaloids on Purified Myrosinase from Lepidium sativum Seedlings". Plant Physiol. 90 (1): 48–52. doi:10.1104/pp.90.1.48. PMC 1061675. PMID 16666767.
- Ohtsuru, M.; Kawatani, H. (1979). "Studies on the myrosinase from Wasabia japonica: Purification and some properties of wasabi myrosinase". Agric. Biol. Chem. 43 (11): 2249–2255. doi:10.1271/bbb1961.43.2249.
- Iversen, T.-H.; Baggerud, C. (1980). "Myrosinase activity in differentiated and undifferentiated plants of Brassiaceae Z.". Z. Pflanzenphysiol. 97 (5): 399–407. doi:10.1016/s0044-328x(80)80014-6.
- El-Sayed, Sanaa T.; Jwanny, Etidal W.; Rashad, Mona M.; Mahmoud, Abeer E.; Abdallah, Nadia M. (1995). "Glycosidases in plant tissues of some brassicaceae screening of different cruciferous plants for glycosidases production". Applied Biochemistry and Biotechnology. 55 (3): 219–230. doi:10.1007/BF02786861. ISSN 0273-2289.
- Ohtsuru, M.; Hata, T. (1972). "Molecular Properties of Multiple Forms of Plant Myrosinase". Agric. Biol. Chem. 36 (13): 2495–2503. doi:10.1271/bbb1961.36.2495.
- Lonnerdal, B.; Janson, J.-C. (1973). "Studies on myrosinases. II. Purification and characterization of a myrosinase from rapeseed (Brassica napus L.)". Biochim. Biophys. Acta. 315 (2): 421–429. doi:10.1016/0005-2744(73)90272-6.
- Nakamura Yoshimasa (2007). "Papaya Seed Represents a Rich Source of Biologically Active Isothiocyanate". Journal of Agricultural and Food Chemistry. 55 (11): 4407–4413. doi:10.1021/jf070159w. PMID 17469845.
- Husebye, H. (2005). "Crystal structure at 1.1 Å resolution of an insect myrosinase from Brevicoryne brassicae shows its close relationship to β-glucosidases". Insect Biochemistry and Molecular Biology. 35 (12): 1311–1320. doi:10.1016/j.ibmb.2005.07.004. PMID 16291087.
- Bridges, M.; et al. (2002). "Spatial organization of the glucosinolate–myrosinase system in brassica specialist aphids is similar to that of the host plant". Proceedings of the Royal Society. 269 (1487): 187–191. doi:10.1098/rspb.2001.1861. PMC 1690872. PMID 11798435.
- Brabban, A. D.; Edwards, C. (1994). "Isolation of glucosinolate degrading microorganisms and their potential for reducing the glucosinolate content of rapemeal". FEMS Microbiology Letters. 119 (1–2): 83–88. doi:10.1111/j.1574-6968.1994.tb06871.x. PMID 8039675.
- Bones, A. M.; Rossiter, J. T. (1996). "The myrosinase-glucosinolate system, its organisation and biochemistry". Physiologia Plantarum. 97: 194–208. doi:10.1111/j.1399-3054.1996.tb00497.x.
- Hayes, J.; Kelleher, M. O.; Eggleston, I. M. (2008). "The Cancer Chemopreventetive Actions of Phytochemicals Derived From Glucosinolates". European Journal of Nutrition. 47: 73–88. doi:10.1007/s00394-008-2009-8. PMID 18458837.
- Ahn, Y.-H.; et al. (2010). "Electrophilic tuning of the chemoprotective natural product sulforaphane". PNAS. 107 (21): 9590–9595. doi:10.1073/pnas.1004104107. PMC 2906893. PMID 20439747.
- Cheng, D.-L.; Hashimoto, K.; Uda, Y. (2004). "In vitro digestion of sinigrin and glucotropaeolin by single strains of Bifidobacterium and identification of the digestive products". Food and Chemical Toxicology. 42 (3): 351–357. doi:10.1016/j.fct.2003.09.008. PMID 14871576.
- Elfoul, L.; et al. (2001). "Formation of allyl isothiocyanate from sinigrin in the digestive tract of rats monoassociated with a human colonic strain of Bacteroides thetaiotaomicron". FEMS Microbiology Letters. 197 (1): 99–103. doi:10.1111/j.1574-6968.2001.tb10589.x. PMID 11287153.
- Chu M, Seltzer TF (2010). "Myxedema coma induced by ingestion of raw bok choy". The New England Journal of Medicine. 362 (20): 1945–6. doi:10.1056/NEJMc0911005. PMID 20484407.