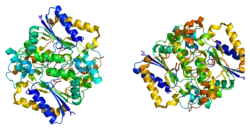NAD(P)H dehydrogenase (quinone 1)
NAD(P)H dehydrogenase [quinone] 1 is an enzyme that in humans is encoded by the NQO1 gene.[5] This protein-coding gene is a member of the NAD(P)H dehydrogenase (quinone) family and encodes a 2-electron reductase (enzyme). This FAD-binding protein forms homodimers and performs two-electron reduction of quinones to hydroquinones and of other redox dyes. It has a preference for short-chain acceptor quinones, such as ubiquinone, benzoquinone, juglone and duroquinone.[6] This gene has an important paralog NQO2. This protein is located in the cytosol.[7]
NQO1 enzyme expression can be induced by dioxin[8] and inhibited by dicoumarol.[9]
Function
This gene is a member of the NAD(P)H dehydrogenase (quinone) family and encodes a cytoplasmic 2-electron reductase. This FAD-binding protein forms homodimers and reduces quinones to hydroquinones. This protein's enzymatic activity prevents the one electron reduction of quinones that results in the production of radical species.[10]
The ubiquitin-independent p53 degradation pathway is regulated by NQO1. NQO1 stabilizes p53, protecting it from degradation. Individuals with decreased NQO1 expression/activity have reduced p53 stability, which may lead to resistance to drugs such as chemotherapeutics.[11]
Detoxification
Quinonoid compounds generate reactive oxygen species (ROS) via redox cycling mechanisms and arylating nucleophiles. NQO1 is employed in the removal of a quinone from biological systems as a detoxification reaction: NAD(P)H + a quinone → NAD(P)+ + a hydroquinone. This reaction ensures complete oxidation of the substrate without the formation of semiquinones and species with reactive oxygen radicals that are deleterious to cells. The localization of NQO1 in epithelial and endothelial tissues of mice, rats and humans indicates their importance in detoxifying agent, since their location facilitates exposure to compounds entering the body.
Vitamin K metabolism
The enzyme is also involved in biosynthetic processes such as the vitamin K-dependent gamma-carboxylation of glutamate residues in prothrombin synthesis.[12] NQO1 catalyzes the reduction of vitamin K1,K2 and K3 into their hydroquinone form, but it only has a high affinity for Vitamin K3. Vitamin K hydroquinone serves as a cofactor for vitamin K γ‐carboxylase that catalyzes γ‐carboxylation of specific glutamic acid residues in Gla‐factors/proteins (Gla domain) leading to their activation and participation in blood clotting and bone metabolism. Vitamin K is used as radiation sensitizer or in mixtures with other chemotherapeutic drugs to treat several types of cancer. ROS generated in redox cycling contributes to anticancer activity of vitamin K. NQO1 competes with enzymes that redox cycle vitamin K to formation of semiquinone and ROS. NQO1is therefore able to detoxify vitamin K3 and protect cells against oxidative stress.[13]
Bioactivation of antitumor agents
Several anti-tumor agents such as mitosenes, indolequinones, aziridinylbenzoquinones and β-lapachone have been designed be bioactivated by NQO1 from various prodrugs. The high levels of NQO1 expression in many human solid tumors compared to normal tissue ensures their selective activation within tumor cells.[14][15]
Reduction of endogenous quinones
NQO1 plays a role in ubiquinone and vitamin E quinone metabolism. These quinones protect cellular membranes from peroxidative injury in their reduced state. Furthermore, reduced forms of ubiquinone and vitamin E quinone have been shown to possess antioxidant properties that are superior to their non-reduced forms.[16]
Polymorphisms
P187S
One widespread single-nucleotide polymorphism of the NQO1 gene (NQO1*2), found homozygous in 4% to 20% of different populations, has found to be connected with different forms of cancer and a lowered efficiency of some chemotherapeutics like mitomycin C. This single nucleotide polymorphism leads to a proline serine exchange on position 187. NAD(P)H dehydrogenase [quinone] 1 P187S has been shown to have a lowered activity and stability. Crystallographic and nuclear magnetic resonance data show that the reason for this different behaviour is found in a flexible C-terminus of the protein leading to a destabilization of the whole protein.[17] Recent pharmacological research suggests feasibility of genotype-directed redox chemotherapeutic intervention targeting NQO1*2 breast cancer.[18]
A comprehensive meta-analysis showed an association between overall cancer risk and P187S.[19]
R139W
One further single nucleotide polymorphism, found homozygous in 0% to 5% of different ethnic population, is leading to an amino acid exchange on position 139 from arginine to tryptophane.[20] Furthermore, an alternative RNA splicing site is created leading to a loss of the quinone binding site.[21] The variant protein of NQO1*3 has similar stability as its wild-type counterpart. The variation between the two is substrate specific and it has reduced activity for some substrates.[22] It has been recently shown that the NQO1*3 polymorphism may also lead to reduced NQO1 protein expression.[11]
Interactions
NAD(P)H dehydrogenase (quinone 1) has been shown to interact with HSPA4,[23] p53, p33 and p73.[17]
Regulation by Keap1/Nrf2/ARE pathway
External (via chemicals) and internal (stress response or caloric restriction) induction of NQO1 is mediated solely through the Keap1/Nrf2/ARE. Keap1 acts as the sensor which loses its ability to target Nrf2 for degradation upon exposure to the inducers. Nrf2 is consequently stabilized and accumulated in the nucleus upon which it binds to the AREs and initiates expression of cytoprotective genes including NQO1.[24]
p53 and p73
p53 and p73 are tumor suppressor proteins and their degradation is tightly regulated by ubiquitination. Recently it was shown that their degradation can also occur via an ubiquitin-independent process;[25] NQO1 blocks p53 and p73 degradation in the presence of NADH and protects them from 20S proteasomal degradation. This protein-protein interaction between p53 and NQO1 was non-catalytic.[26]
Ornithine decarboxylase
Ornithine decarboxylase (ODC), is a labile protein that is the first rate-limiting enzyme in polyamine biosynthesis. Its degradation is regulated by antizyme that is induced by polyamine production. NQO1 has been shown to stabilize the degradation of ODC by binding to it and protecting it from 20S proteasomal degradation.
Clinical significance
Mutations in this gene have been associated with tardive dyskinesia (TD), an increased risk of hematotoxicity after exposure to benzene, and susceptibility to various forms of cancer. Altered expression of this protein has been seen in many tumors and is also associated with Alzheimer's disease (AD).[10]
Benzene toxicity
Benzene poisoning can increase risk of hematological cancers and other disorders. The mechanism of benzene metabolism and how it affects toxicity has not been completely understood. A general observation is that there is high variation in the extent of damage due to benzene poisoning. A possible explanation is the accumulation of phenols and hydroquinone in the target organ—the bone marrow—and subsequent oxidation of these metabolites to reactive quinone metabolites via a number of possible pathways.[11] A case-control study conducted in China showed that patients with two copies of the NQO1 C609T (NQO1*2 polymorphism) mutation had a 7.6-fold increased risk of benzene poisoning compared to those who carried one or two wild-type NQO1 alleles.[27]
Alzheimer's disease
Oxidative stress has been linked to onset of Alzheimer's disease (AD)[28] Since the NQO1*2 polymorphism affects the NQO1 activity and hence increase in oxidative stress, it has been postulated that this might increase the susceptibility of affected subjects for developing AD. A study conducted with a Chinese population consisting of 104 LOAD patients and 128 control patients disproved this hypothesis.[29]
Cancer
Meta-analyses have been performed to examine the association between NQO1 polymorphism and increased cancer risk.[19] The results from some of these analyses have been summarized in the table below:
| Cancer Type | Polymorphism | Risk Odds Ratio (95% Confidence Interval) | Reference |
|---|---|---|---|
| Prostate | C609T | All ethnicities: No significant change
Asians: 1.419 (1.1053-1.913) |
[30] |
| Acute Lymphoblastic Leukemia | C609T | All ethnicities: 1.46 (1.18-1.79)
Non-Asians 1.74 (1.29-2.36) |
[31] |
| Breast | C609T | All ethnicities: No significant change
Caucasians: 1.177 (1.041-1.331) |
[32] |
| Colorectal | C609T | All ethnicities: 1.34 (1.10-1.64) | [33] |
| Bladder | C609T | All ethnicities: 1.18 (1.06-1.31) | [34] |
| De novo childhood leukemia | C609T | All ethnicities: 1.58 (1.22-2.07)
Europeans, Asians: 1.52 (1.05-2.19)
|
[35] |
References
- GRCh38: Ensembl release 89: ENSG00000181019 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000003849 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Jaiswal AK, McBride OW, Adesnik M, Nebert DW (1988). "Human dioxin-inducible cytosolic NAD(P)H:menadione oxidoreductase. cDNA sequence and localization of gene to chromosome 16". J. Biol. Chem. 263 (27): 13572–8. PMID 2843525.
- Sparla F, Tedeschi G, Trost P (Sep 1996). "NAD(P)H:(Quinone-Acceptor) Oxidoreductase of Tobacco Leaves Is a Flavin Mononucleotide-Containing Flavoenzyme". Plant Physiology. 112 (1): 249–258. doi:10.1104/pp.112.1.249. PMC 157943. PMID 12226388.
- "NQO1 localizations". COMPARTMENTS.
- Jaiswal AK (Nov 1991). "Human NAD(P)H:quinone oxidoreductase (NQO1) gene structure and induction by dioxin". Biochemistry. 30 (44): 10647–53. doi:10.1021/bi00108a007. PMID 1657151.
- Arlt VM, Stiborova M, Henderson CJ, Osborne MR, Bieler CA, Frei E, Martinek V, Sopko B, Wolf CR, Schmeiser HH, Phillips DH (Apr 2005). "Environmental pollutant and potent mutagen 3-nitrobenzanthrone forms DNA adducts after reduction by NAD(P)H:quinone oxidoreductase and conjugation by acetyltransferases and sulfotransferases in human hepatic cytosols". Cancer Research. 65 (7): 2644–52. doi:10.1158/0008-5472.CAN-04-3544. PMID 15805261.
- "Entrez Gene: NQO1 NAD(P)H dehydrogenase, quinone 1".
- Ross D, Siegel D (2004). "NAD(P)H:quinone oxidoreductase 1 (NQO1, DT-diaphorase), functions and pharmacogenetics". Quinones and Quinone Enzymes, Part B. Methods in Enzymology. 382. pp. 115–44. doi:10.1016/S0076-6879(04)82008-1. ISBN 9780121827861. PMID 15047100.
- "P15559 - NQO1_HUMAN".
- Gong X (2008). "Quinone Oxidoreductases and Vitamin K Metabolism". Vitamin K. Vitamins and Hormones. 78. Academic Press. pp. 85–101. doi:10.1016/S0083-6729(07)00005-2. ISBN 978-0-12-374113-4. PMID 18374191.
- Ross D, Kepa JK, Winski SL, Beall HD, Anwar A, Siegel D (Dec 2000). "NAD(P)H:quinone oxidoreductase 1 (NQO1): chemoprotection, bioactivation, gene regulation and genetic polymorphisms". Chemico-Biological Interactions. 129 (1–2): 77–97. doi:10.1016/S0009-2797(00)00199-X. PMID 11154736.
- Dong GZ, Oh ET, Lee H, Park MT, Song CW, Park HJ (May 2010). "Beta-lapachone suppresses radiation-induced activation of nuclear factor-kappaB". Experimental & Molecular Medicine. 42 (5): 327–34. doi:10.3858/emm.2010.42.5.034. PMC 2877251. PMID 20200474.
- Kohar I, Baca M, Suarna C, Stocker R, Southwell-Keely PT (Aug 1995). "Is alpha-tocopherol a reservoir for alpha-tocopheryl hydroquinone?". Free Radical Biology & Medicine. 19 (2): 197–207. doi:10.1016/0891-5849(95)00010-U. PMID 7649491.
- Lienhart WD, Gudipati V, Uhl MK, Binter A, Pulido SA, Saf R, Zangger K, Gruber K, Macheroux P (2014). "Collapse of the native structure caused by a single amino acid exchange in human NAD(P)H:quinone oxidoreductase". FEBS J. 281 (20): 4691–4704. doi:10.1111/febs.12975. PMC 4612375. PMID 25143260.
- Cabello CM, Lamore SD, Bair WB, Davis AL, Azimian SM, Wondrak GT (2011). "DCPIP (2,6-dichlorophenolindophenol) as a genotype-directed redox chemotherapeutic targeting NQO1*2 breast carcinoma". Free Radic. Res. 45 (3): 276–292. doi:10.3109/10715762.2010.526766. PMC 4101082. PMID 21034357.
- Lajin B, Alachkar A (Sep 2013). "The NQO1 polymorphism C609T (Pro187Ser) and cancer susceptibility: a comprehensive meta-analysis". British Journal of Cancer. 109 (5): 1325–37. doi:10.1038/bjc.2013.357. PMC 3778271. PMID 23860519.
- Dinkova-Kostova AT, Talalay P (2010). "NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector" (PDF). Arch. Biochem. Biophys. 501 (1): 116–23. doi:10.1016/j.abb.2010.03.019. PMC 2930038. PMID 20361926.
- Gasdaska PY, Fisher H, Powis G (1995). "An alternatively spliced form of NQO1 (DT-diaphorase) messenger RNA lacking the putative quinone substrate binding site is present in human normal and tumor tissues". Cancer Res. 55 (12): 2542–7. PMID 7780966.
- Pan SS, Forrest GL, Akman SA, Hu LT (Jan 1995). "NAD(P)H:quinone oxidoreductase expression and mitomycin C resistance developed by human colon cancer HCT 116 cells". Cancer Research. 55 (2): 330–5. PMID 7812966.
- Anwar A, Siegel D, Kepa JK, Ross D (2002). "Interaction of the molecular chaperone Hsp70 with human NAD(P)H:quinone oxidoreductase 1". J. Biol. Chem. 277 (16): 14060–7. doi:10.1074/jbc.M111576200. PMID 11821413.
- Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P (Sep 2002). "Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants". Proceedings of the National Academy of Sciences of the United States of America. 99 (18): 11908–13. Bibcode:2002PNAS...9911908D. doi:10.1073/pnas.172398899. PMC 129367. PMID 12193649.
- Asher G, Tsvetkov P, Kahana C, Shaul Y (Feb 2005). "A mechanism of ubiquitin-independent proteasomal degradation of the tumor suppressors p53 and p73". Genes & Development. 19 (3): 316–21. doi:10.1101/gad.319905. PMC 546509. PMID 15687255.
- Asher G, Bercovich Z, Tsvetkov P, Shaul Y, Kahana C (Mar 2005). "20S proteasomal degradation of ornithine decarboxylase is regulated by NQO1". Molecular Cell. 17 (5): 645–55. doi:10.1016/j.molcel.2005.01.020. PMID 15749015.
- Rothman N, Smith MT, Hayes RB, Traver RD, Hoener B, Campleman S, Li GL, Dosemeci M, Linet M, Zhang L, Xi L, Wacholder S, Lu W, Meyer KB, Titenko-Holland N, Stewart JT, Yin S, Ross D (Jul 1997). "Benzene poisoning, a risk factor for hematological malignancy, is associated with the NQO1 609C-->T mutation and rapid fractional excretion of chlorzoxazone". Cancer Research. 57 (14): 2839–42. PMID 9230185.
- Miranda S, Opazo C, Larrondo LF, Muñoz FJ, Ruiz F, Leighton F, Inestrosa NC (Dec 2000). "The role of oxidative stress in the toxicity induced by amyloid beta-peptide in Alzheimer's disease". Progress in Neurobiology. 62 (6): 633–48. doi:10.1016/S0301-0082(00)00015-0. hdl:10533/172411. PMID 10880853. S2CID 53185151.
- Wang B, Jin F, Xie Y, Tang Y, Kan R, Zheng C, Yang Z, Wang L (Dec 2006). "Association analysis of NAD(P)H:quinone oxidoreductase gene 609 C/T polymorphism with Alzheimer's disease". Neuroscience Letters. 409 (3): 179–81. doi:10.1016/j.neulet.2006.09.042. PMID 17027152. S2CID 19068892.
- Sun Z, Cui Y, Pei J, Fan Z (Aug 2014). "Association between NQO1 C609T polymorphism and prostate cancer risk". Tumour Biology. 35 (8): 7993–8. doi:10.1007/s13277-014-2051-5. PMID 24838947. S2CID 13964666.
- Li C, Zhou Y (Jun 2014). "Association between NQO1 C609T polymorphism and acute lymphoblastic leukemia risk: evidence from an updated meta-analysis based on 17 case-control studies". Journal of Cancer Research and Clinical Oncology. 140 (6): 873–81. doi:10.1007/s00432-014-1595-5. PMID 24488035. S2CID 33710993.
- Peng Q, Lu Y, Lao X, Chen Z, Li R, Sui J, Qin X, Li S (2014). "The NQO1 Pro187Ser polymorphism and breast cancer susceptibility: evidence from an updated meta-analysis". Diagnostic Pathology. 9: 100. doi:10.1186/1746-1596-9-100. PMC 4041044. PMID 24884893.
- Zheng B, Wang Z, Chai R (Aug 2014). "NQO1 C609T polymorphism and colorectal cancer susceptibility: a meta-analysis". Archives of Medical Science. 10 (4): 651–60. doi:10.5114/aoms.2014.44856. PMC 4175766. PMID 25276147.
- Gong M, Yi Q, Wang W (Oct 2013). "Association between NQO1 C609T polymorphism and bladder cancer susceptibility: a systemic review and meta-analysis". Tumour Biology. 34 (5): 2551–6. doi:10.1007/s13277-013-0799-7. PMID 23749485. S2CID 18272815.
- Yang FY, Guan QK, Cui YH, Zhao ZQ, Rao W, Xi Z (Sep 2012). "NAD(P)H quinone oxidoreductase 1 (NQO1) genetic C609T polymorphism is associated with the risk of digestive tract cancer: a meta-analysis based on 21 case-control studies". European Journal of Cancer Prevention. 21 (5): 432–41. doi:10.1097/CEJ.0b013e32834f7514. PMID 22387672. S2CID 41837215.
Further reading
- Vasiliou V, Ross D, Nebert DW (2006). "Update of the NAD(P)H:quinone oxidoreductase (NQO) gene family". Hum. Genomics. 2 (5): 329–35. doi:10.1186/1479-7364-2-5-329. PMC 3500182. PMID 16595077.
- Li Y, Jaiswal AK (1992). "Regulation of human NAD(P)H:quinone oxidoreductase gene. Role of AP1 binding site contained within human antioxidant response element". J. Biol. Chem. 267 (21): 15097–104. PMID 1340765.
- Jaiswal AK (1991). "Human NAD(P)H:quinone oxidoreductase (NQO1) gene structure and induction by dioxin". Biochemistry. 30 (44): 10647–53. doi:10.1021/bi00108a007. PMID 1657151.
- Traver RD, Horikoshi T, Danenberg KD, Stadlbauer TH, Danenberg PV, Ross D, Gibson NW (1992). "NAD(P)H:quinone oxidoreductase gene expression in human colon carcinoma cells: characterization of a mutation which modulates DT-diaphorase activity and mitomycin sensitivity". Cancer Res. 52 (4): 797–802. PMID 1737339.
- Chen LZ, Harris PC, Apostolou S, Baker E, Holman K, Lane SA, Nancarrow JK, Whitmore SA, Stallings RL, Hildebrand CE (1991). "A refined physical map of the long arm of human chromosome 16". Genomics. 10 (2): 308–12. doi:10.1016/0888-7543(91)90313-4. PMID 2071140.
- Maruyama K, Sugano S (1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Rothman N, Smith MT, Hayes RB, Traver RD, Hoener B, Campleman S, Li GL, Dosemeci M, Linet M, Zhang L, Xi L, Wacholder S, Lu W, Meyer KB, Titenko-Holland N, Stewart JT, Yin S, Ross D (1997). "Benzene poisoning, a risk factor for hematological malignancy, is associated with the NQO1 609C-->T mutation and rapid fractional excretion of chlorzoxazone". Cancer Res. 57 (14): 2839–42. PMID 9230185.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Smiley JF, Levey AI, Mesulam MM (1998). "Infracortical interstitial cells concurrently expressing m2-muscarinic receptors, acetylcholinesterase and nicotinamide adenine dinucleotide phosphate-diaphorase in the human and monkey cerebral cortex". Neuroscience. 84 (3): 755–69. doi:10.1016/S0306-4522(97)00524-1. PMID 9579781. S2CID 25807845.
- Moran JL, Siegel D, Ross D (1999). "A potential mechanism underlying the increased susceptibility of individuals with a polymorphism in NAD(P)H:quinone oxidoreductase 1 (NQO1) to benzene toxicity". Proc. Natl. Acad. Sci. U.S.A. 96 (14): 8150–5. Bibcode:1999PNAS...96.8150M. doi:10.1073/pnas.96.14.8150. PMC 22203. PMID 10393963.
- Kristiansen OP, Larsen ZM, Johannesen J, Nerup J, Mandrup-Poulsen T, Pociot F (1999). "No linkage of P187S polymorphism in NAD(P)H: quinone oxidoreductase (NQO1/DIA4) and type 1 diabetes in the Danish population. DIEGG and DSGD. Danish IDDM Epidemiology and Genetics Group and The Danish Study Group of Diabetes in Childhood". Hum. Mutat. 14 (1): 67–70. doi:10.1002/(SICI)1098-1004(1999)14:1<67::AID-HUMU8>3.0.CO;2-5. PMID 10447260.
- Eliasson M, Boström M, DePierre JW (1999). "Levels and subcellular distributions of detoxifying enzymes in the ovarian corpus luteum of the pregnant and non-pregnant pig". Biochem. Pharmacol. 58 (8): 1287–92. doi:10.1016/S0006-2952(99)00185-9. PMID 10487530.
- Skelly JV, Sanderson MR, Suter DA, Baumann U, Read MA, Gregory DS, Bennett M, Hobbs SM, Neidle S (1999). "Crystal structure of human DT-diaphorase: a model for interaction with the cytotoxic prodrug 5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB1954)". J. Med. Chem. 42 (21): 4325–30. doi:10.1021/jm991060m. PMID 10543876.
- Faig M, Bianchet MA, Talalay P, Chen S, Winski S, Ross D, Amzel LM (2000). "Structures of recombinant human and mouse NAD(P)H:quinone oxidoreductases: species comparison and structural changes with substrate binding and release". Proc. Natl. Acad. Sci. U.S.A. 97 (7): 3177–82. doi:10.1073/pnas.050585797. PMC 16212. PMID 10706635.
- Harada S, Fujii C, Hayashi A, Ohkoshi N (2001). "An association between idiopathic Parkinson's disease and polymorphisms of phase II detoxification enzymes: glutathione S-transferase M1 and quinone oxidoreductase 1 and 2". Biochem. Biophys. Res. Commun. 288 (4): 887–92. doi:10.1006/bbrc.2001.5868. PMID 11688992.
- Siegel D, Ryder J, Ross D (2001). "NAD(P)H: quinone oxidoreductase 1 expression in human bone marrow endothelial cells". Toxicol. Lett. 125 (1–3): 93–8. doi:10.1016/S0378-4274(01)00426-X. PMID 11701227.
- Anwar A, Siegel D, Kepa JK, Ross D (2002). "Interaction of the molecular chaperone Hsp70 with human NAD(P)H:quinone oxidoreductase 1". J. Biol. Chem. 277 (16): 14060–7. doi:10.1074/jbc.M111576200. PMID 11821413.
- Winski SL, Koutalos Y, Bentley DL, Ross D (2002). "Subcellular localization of NAD(P)H:quinone oxidoreductase 1 in human cancer cells". Cancer Res. 62 (5): 1420–4. PMID 11888914.
- Begleiter A, Lange L (2002). "Lack of NQO1 induction in human tumor cells is not due to changes in the promoter region of the gene". Int. J. Oncol. 20 (4): 835–8. doi:10.3892/ijo.20.4.835. PMID 11894133.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.