Neotame
Neotame, also known by the trade name Newtame,[3] is a non-caloric artificial sweetener and aspartame analog by NutraSweet.[2] By mass, it is 8000 times sweeter than sucrose.[4] It has no notable off-flavors when compared to sucrose. It enhances original food flavors. It can be used alone, but is often mixed with other sweeteners to increase their individual sweetness (i.e. synergistic effect) and decrease their off-flavors (e.g. saccharin). It is chemically somewhat more stable than aspartame. Its use can be cost effective in comparison to other sweeteners as smaller amounts of neotame are needed.[2]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(3S)-3-(3,3-Dimethylbutylamino)-4-[[(2S)-1-methoxy-1-oxo-3-phenylpropan-2-yl]amino]-4-oxobutanoic acid | |
| Other names
E961; N-(N-(3,3-Dimethylbutyl)-L-α-aspartyl)-L-phenylalanine 1-methyl ester | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.109.344 |
| E number | E961 (glazing agents, ...) |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C20H30N2O5 | |
| Molar mass | 378.469 g·mol−1 |
| Appearance | white powder[1] |
| Melting point | 80.9–83.4 °C (177.6–182.1 °F; 354.0–356.5 K)[1] |
| 12.6 g/kg at 25 °C[2] | |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It is suitable for use in carbonated soft drinks, yogurts, cakes, drink powders, bubble gums among other foods. It can be used as a table top sweetener for hot drinks like coffee. It covers bitter tastes (e.g. caffeine).[2]
In 2002, FDA approved it as a non-nutritive sweetener and flavor enhancer within United States in foods generally, except meat and poultry.[3] In 2010, it was approved for use in foods within EU with the E number E961.[5] It has also been approved as an additive in many other countries outside US and EU.[2]
Its metabolism is fast and it does not retain in the body. Methanol forms in its metabolism. Only trace amounts of neotame are added to foods, so the amount of methanol is insignificant for health. It is safe for type 2 diabetics and those with phenylketonuria.[6][1]
French scientists Claude Nofre and Jean-Marie Tinti invented neotame.[2] In 1992 they filed a patent for neotame within US, which was granted in 1996.[7]
Safety
In US and EU, the acceptable daily intake (ADI) of neotame for humans is 0.3 and 2 mg per kg of bodyweight (mg/kg bw), respectively. NOAEL for humans is 200 mg/kg bw per day within EU.[3][1] Estimated possible daily intakes from foods are well below ADI-levels. Ingested neotame can form phenylalanine, but in normal use of neotame, this is not significant to those with phenylketonuria. It also has no adverse effects in type 2 diabetics. It is not considered to be carcinogenic or mutagenic.[6][1]
The Center for Science in the Public Interest ranks neotame as safe.[8]
Sweetness
Neotame is sweet because it binds to TAS1R2-receptors of mouth as an agonist. Aspartame binds to the same receptor.[9]
Water solutions of neotame, that are equivalent in sweetness to sucrose water solutions, increase logarithmically in relative sweetness as the sucrose concentration of a comparably sweet sucrose solution increases, until a plateau is reached. Maximum sweetness is reached at neotame solution concentrations that are relatively as sweet as a water solution that is 15.1 percentage sucrose by weight, i.e. at 15.1 sucrose equivalence % (SE%). For comparison, acesulfame K, cyclamate and saccharin reach their maximum sweetness at 11.6 SE%, 11.3 SE% and 9 SE%, respectively.[2]
Chemistry
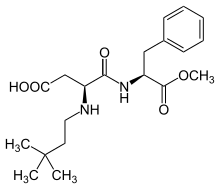
Structure
Neotame is formally a secondary amine of 3,3-dimethylbutanal and aspartame. Latter is a dipeptide of phenylalanine and aspartic acid. Neotame has 2 stereocenters and 4 stereoisomers. Sweetness is due to the (2S),(3S)-stereoisomer.[10]
Properties and reactivity

Neotame has similar stability as aspartame, but has greater stability especially in heated and dairy foods. Increased temperature, moisture or pH increase losses, and are the main relevant properties of a food when considering the stability of neotame. For example, about 90% of original neotame remains after 8 weeks of storage in pH 3.2 beverages. Neotame is especially stable as a dry powder at room temperature and humidity even if mixed with e.g. glucose or maltodextrin, and is relatively inert in foods with reducing sugars like fructose.[2]
Unlike aspartame, neotame doesn't form diketopiperazines via intra-molecular cyclization due to its N-alkyl substitution with 3,3-dimethylbutyl. This increases its heat stability.[2]
Over 1000 g of neotame dissolves in 1 kg of ethanol at 15 °C. At 15 °C the solubility of neotame is 10.6 g/kg in water and 43.6 g/kg in ethyl acetate. At 25 °C the solubilities are 12.6 g/kg and 77.0 g/kg, respectively. At 40 °C the solubilities are 18.0 g/kg and 238 g/kg, respectively. At 50 °C the solubilities are 25.2 g/kg and 872 g/kg, respectively.[2] Neotame is acidic and its 0.5 wt% solution has a pH of 5.80.[1]
Manufacture
Industrially neotame is made from 3,3-dimethylbutanal and aspartame via reductive amination.[2] They are dissolved in methanol, palladium on carbon catalyst is added, air is replaced with hydrogen and the reaction is carried out in room temperature under pressure for a few hours. Catalyst is filtered out. This can be aided with diatomaceous earth. Methanol is distilled followed by addition of water. The mixture is cooled for a few hours, neotame is isolated via centrifugation, washed with water and vacuum dried. Neotame is milled to suitable size.[1]
Metabolism
In humans and many other animals like dogs, rats and rabbits, neotame is rapidly, but incompletely absorbed. It or its metabolites are not retained or concentrated in specific tissues.[1]

In humans, at oral doses of about 0.25 mg per kg of bodyweight (mg/kg bw), about 34% is absorbed to blood. Pharmacokinetics of oral doses of 0.1–0.5 mg/kg bw are somewhat linear, and at such doses, maximum neotame concentration in blood plasma is reached after about 0.5 hours with a half-life of about 0.75 hours. In blood and in body in general, non-specific esterases degrade neotame to de-esterified neotame and methanol, which is the main metabolic pathway in humans. De-esterified neotame has a plasma half-life of about 2 hours, and is the main metabolite in plasma.[1]
In humans, over 80% of the original oral dose is excreted in feces and urine within 48 hours and the rest later. About 64% of the original dose is excreted in feces mostly as metabolites. Major metabolite in feces is the de-esterified neotame. Over 1% of the original dose is excreted in feces as N-(3,3-dimethylbutyl)-L-aspartic acid. Over 1% is excreted in urine as carnitine conjugate of 3,3-dimethylbutyric acid. Other minor metabolites form.[1]
Methanol of neotame metabolism is insignificant at regulated levels used in foods and in comparison to methanol naturally found in foods. Nitrosation of neotame to nitroso compounds is hypothetical. It is also considered insignificant even it occurs, as plausible nitroso neotame analogs have been synthesized and shown in Ames tests to not to be mutagenic.[1]
Patent
The patent covering the neotame molecule in the US, 5,480,668,[7] was originally set to expire 7 November 2012, but was extended 973 days by the U.S. Patent and Trademark Office. The patent expired on 8 July 2015.[11]
Notes
- "Neotame as a sweetener and flavour enhancer - Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food". EFSA Journal. 5 (11): 581. 2007. doi:10.2903/j.efsa.2007.581. ISSN 1831-4732.
- Nabors 2012, p. 134–149
- Nutrition (2019-02-09). "Additional Information about High-Intensity Sweeteners Permitted for Use in Food in the United States". FDA.
- Nabors 2012, p. 2–3
- Halliday, Jess. "Neotame wins approval in Europe". foodnavigator.com. Retrieved 2019-09-15.
- "Food additives permitted for direct addition to food for human consumption; neotame" (PDF). Federal Register. 67 (131): 45300–45310. 2002.
- "US 5,480,668". Retrieved 2019-09-15.
- "Chemical Cuisine | Center for Science in the Public Interest". cspinet.org. Retrieved 2019-09-15.
- Assadi-Porter FM, et al. (2018). "Multimodal Ligand Binding Studies of Human and Mouse G-Coupled Taste Receptors to Correlate Their Species-Specific Sweetness Tasting Properties". Molecules. 23 (10): 2531. doi:10.3390/molecules23102531. ISSN 1420-3049. PMC 6214618. PMID 30282936.
- Bathinapatla A, et al. (2014). "Determination of Neotame by High-Performance Capillary Electrophoresis Using ß-cyclodextrin as a Chiral Selector". Analytical Letters. 47 (17): 2795–2812. doi:10.1080/00032719.2014.924008. ISSN 0003-2719. S2CID 93160173.
- "USPTO extension of 5,480,668". Retrieved 2012-09-21.
References
- Nabors LO, et al. (2012). Alternative sweeteners (4th ed.). CRC Press. ISBN 978-1439846155. OCLC 760056415.
External links
 Media related to Neotame at Wikimedia Commons
Media related to Neotame at Wikimedia Commons- Official Neotame Website

