Nicotinamide mononucleotide
Nicotinamide mononucleotide ("NMN" and "β-NMN") is a nucleotide derived from ribose and nicotinamide.[1] Like nicotinamide riboside, NMN is a derivative of niacin, and humans have enzymes that can use NMN to generate nicotinamide adenine dinucleotide (NADH).[1] In mice, NMN enters cells via the small intestines within 10 minutes converting to NAD+ through the Slc12a8 NMN transporter.[2]
 | |
| Names | |
|---|---|
| IUPAC name
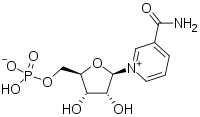
3-Carbamoyl-1-[5-O-(hydroxyphosphinato)-β-D-ribofuranosyl]pyridinium | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| 3570187 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.012.851 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C11H15N2O8P | |
| Molar mass | 334.221 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Because NADH is a cofactor for processes inside mitochondria, for sirtuins, and for PARP, NMN has been studied in animal models as a potential neuroprotective and anti-aging agent.[3][4] Dietary supplement companies have aggressively marketed NMN products claiming those benefits.[5] Doses of up to 500 mg was shown safe in men in a recent human study at Keio University School of Medicine, Shinjuku, Tokyo Japan.[6]
Nicotinamide riboside (NR) kinase enzymes are essential for exogenously administered utilization of NR and NMN.[7][8] Some research suggests when administered exogenously, NMN must be converted to NR in order to enter a cell and be re-phosphorylated back to NMN.[7]
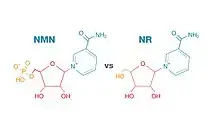
The molecular structures of NMN and NR are roughly the same, except NMN has an added phosphate group. This added phosphate group makes NMN a larger molecule than NR. Some scientists believe NMN is too large to cross cellular membranes and must convert to NR before entering cells, where NAD+ biosynthesis occurs. Otherwise, NMN would need to get transported into cells by a transporter specific for NMN, such as Slc12a8.[9]
Both NR and NMN are vulnerable to extracellular degradation by CD38 enzyme,[8] which can be inhibited by compounds such as CD38-IN-78c.[10]
References
- Bogan KL, Brenner C (2008). "Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition". Annual Review of Nutrition. 28: 115–30. doi:10.1146/annurev.nutr.28.061807.155443. PMID 18429699.
- "Slc12a8 is a nicotinamide mononucleotide transporter". Nature. January 2019.
- Brazill JM, Li C, Zhu Y, Zhai RG (June 2017). "+ synthase… It's a chaperone… It's a neuroprotector". Current Opinion in Genetics & Development. 44: 156–162. doi:10.1016/j.gde.2017.03.014. PMC 5515290. PMID 28445802.
- "Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice". Cell Metabolism. 13 December 2016.
- Stipp D (March 11, 2015). "Beyond Resveratrol: The Anti-Aging NAD Fad". Scientific American Blog Network.
- Irie, Junichiro; Inagaki, Emi; Fujita, Masataka; Nakaya, Hideaki; Mitsuishi, Masanori; Yamaguchi, Shintaro; Yamashita, Kazuya; Shigaki, Shuhei; Ono, Takashi; Yukioka, Hideo; Okano, Hideyuki (2020). "Effect of oral administration of nicotinamide mononucleotide on clinical parameters and nicotinamide metabolite levels in healthy Japanese men". Endocrine Journal. 67 (2): 153–160. doi:10.1507/endocrj.EJ19-0313. ISSN 0918-8959.
- Fletcher RS, Lavery GG (October 2018). "The emergence of the nicotinamide riboside kinases in the regulation of NAD+ metabolism". Journal of Molecular Endocrinology. 61 (3): R107–R121. doi:10.1530/JME-18-0085. PMC 6145238. PMID 30307159.
- Cambronne XA, Kraus WL (October 2020). "+ Synthesis and Functions in Mammalian Cells". Trends in Biochemical Sciences. 45 (10): 858–873. doi:10.1016/j.tibs.2020.05.010. PMC 7502477. PMID 32595066.
- "NMN vs NR: The Differences Between These 2 NAD+ Precursors". www.nmn.com. Retrieved 2021-01-11.
- Tarragó MG, Chini CC, Kanamori KS, Warner GM, Caride A, de Oliveira GC, et al. (May 2018). "+ Decline". Cell Metabolism. 27 (5): 1081–1095.e10. doi:10.1016/j.cmet.2018.03.016. PMC 5935140. PMID 29719225.