Phosphorylation
In chemistry, phosphorylation of a molecule is the attachment of a phosphoryl group. This process and its inverse, dephosphorylation, are critical for many cellular processes in biology. Protein phosphorylation is especially important for their function; for example, this modification activates (or deactivates) almost half of the enzymes present in Saccharomyces cerevisiae, thereby regulating their function.[1][2][3] Many proteins (between 1/3 to 2/3 of the proteome in eukaryotes[4][5]) are phosphorylated temporarily, as are many sugars, lipids, and other biologically-relevant molecules.
Glucose
Phosphorylation of sugars is often the first stage in their catabolism. Phosphorylation allows cells to accumulate sugars because the phosphate group prevents the molecules from diffusing back across their transporter. Phosphorylation of glucose is a key reaction in sugar metabolism because many sugars are first converted to glucose before they are metabolized further.
The chemical equation for the conversion of D-glucose to D-glucose-6-phosphate in the first step of glycolysis is given by
- D-glucose + ATP → D-glucose-6-phosphate + ADP
- ΔG° = −16.7 kJ/mol (° indicates measurement at standard condition)
Researcher D. G. Walker of the University of Birmingham determined the presence of two specific enzymes in adult guinea pig liver, both of which catalyze the phosphorylation of glucose to glucose 6 phosphate. The two enzymes have been identified as a specific glucokinase (ATP-D-glucose 6-phosphotransferase) and non-specific hexokinase (ATP-D-hexose 6-phosphotransferase).
Hepatic cells are freely permeable to glucose, and the initial rate of phosphorylation of glucose is the rate-limiting step in glucose metabolism by the liver (ATP-D-glucose 6-phosphotransferase) and non-specific hexokinase (ATP-D-hexose 6-phosphotransferase).[6]
The role of glucose 6-phosphate in glycogen synthase: High blood glucose concentration causes an increase in intracellular levels of glucose 6 phosphate in liver, skeletal muscle and fat (adipose) tissue. (ATP-D-glucose 6-phosphotransferase) and non-specific hexokinase (ATP-D-hexose 6-phosphotransferase). In liver, synthesis of glycogen is directly correlated by blood glucose concentration and in skeletal muscle and adipocytes, glucose has a minor effect on glycogen synthase. High blood glucose releases insulin, stimulating the trans location of specific glucose transporters to the cell membrane.[6][7]
The liver's crucial role in controlling blood sugar concentrations by breaking down glucose into carbon dioxide and glycogen is characterized by the negative delta G value, which indicates that this is a point of regulation with. The hexokinase enzyme has a low Km, indicating a high affinity for glucose, so this initial phosphorylation can proceed even when glucose levels at nanoscopic scale within the blood.
The phosphorylation of glucose can be enhanced by the binding of Fructose-6-phosphate, and lessened by the binding fructose-1-phosphate. Fructose consumed in the diet is converted to F1P in the liver. This negates the action of F6P on glucokinase,[8] which ultimately favors the forward reaction. The capacity of liver cells to phosphorylate fructose exceeds capacity to metabolize fructose-1-phosphate. Consuming excess fructose ultimately results in an imbalance in liver metabolism, which indirectly exhausts the liver cell's supply of ATP.[9]
Allosteric activation by glucose 6 phosphate, which acts as an effector, stimulates glycogen synthase, and glucose 6 phosphate may inhibit the phosphorylation of glycogen synthase by cyclic AMP-stimulated protein kinase.[7]
Phosphorylation of glucose is imperative in processes within the body. For example, phosphorylating glucose is necessary for insulin-dependent mechanistic target of rapamycin pathway activity within the heart. This further suggests a link between intermediary metabolism and cardiac growth.[10]
Glycolysis
Glycolysis is an essential process of glucose degrading into two molecules of pyruvate, through various steps, with the help of different enzymes. It occurs in ten steps and proves that phosphorylation is a much required and necessary step to attain the end products. Phosphorylation initiates the reaction in step 1 of the preparatory step [11] (first half of glycolysis), and initiates step 6 of payoff phase (second phase of glycolysis).[12]
Glucose, by nature, is a small molecule with the ability to diffuse in and out of the cell. By phosphorylating glucose (adding a phosphoryl group in order to create a negatively charged phosphate group[13]), glucose is converted to glucose-6-phosphate and trapped within the cell as the cell membrane is negatively charged. This reaction occurs due to the enzyme hexokinase, an enzyme that helps phosphorylate many six-membered ring structures. Glucose-6-phosphate cannot travel through the cell membrane and is therefore coerced to stay inside the cell. Phosphorylation takes place in step 3, where fructose-6-phosphate is converted to fructose-1,6-bisphosphate. This reaction is catalyzed by phosphofructokinase.
While phosphorylation is performed by ATPs during preparatory steps, phosphorylation during payoff phase is maintained by inorganic phosphate. Each molecule of glyceraldehyde-3-phosphate is phosphorylated to form 1,3-bisphosphoglycerate. This reaction is catalyzed by GAPDH (glyceraldehyde-3-phosphate dehydrogenase). The cascade effect of phosphorylation eventually causes instability and allows enzymes to open the carbon bonds in glucose.
Phosphorylation functions as an extremely vital component of glycolysis, for it helps in transport, control and efficiency.[14]
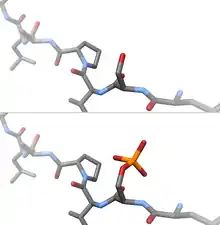
Protein phosphorylation
Protein phosphorylation is considered the most abundant post-translational modification in eukaryotes. Phosphorylation can occur on serine, threonine and tyrosine side chains (often called 'residues') through phosphoester bond formation, on histidine, lysine and arginine through phosphoramidate bonds, and on aspartic acid and glutamic acid through mixed anhydride linkages. Recent evidence confirms widespread histidine phosphorylation at both the 1 and 3 N-atoms of the imidazole ring.[15][16] Recent work demonstrates widespread human protein phosphorylation on multiple non-canonical amino acids, including motifs containing phosphorylated histidine, aspartate, glutamate, cysteine, arginine and lysine in HeLa cell extracts.[17] However, due to the chemical lability of these phosphorylated residues, and in marked contrast to Ser, Thr and Tyr phosphorylation, the analysis of phosphorylated histidine (and other non-canonical amino acids) using standard biochemical and mass spectrometric approaches is much more challenging[17][18][19] and special procedures and separation techniques are required for their preservation alongside classical Ser, Thr and Tyr phosphorylation.[20]
The prominent role of protein phosphorylation in biochemistry is illustrated by the huge body of studies published on the subject (as of March 2015, the MEDLINE database returns over 240,000 articles, mostly on protein phosphorylation).
ATP
ATP, the "high-energy" exchange medium in the cell, is synthesized in the mitochondrion by addition of a third phosphate group to ADP in a process referred to as oxidative phosphorylation. ATP is also synthesized by substrate-level phosphorylation during glycolysis. ATP is synthesized at the expense of solar energy by photophosphorylation in the chloroplasts of plant cells.
See also
- Moiety conservation
- Phosida
- Phosphoamino acid analysis
- RegPhos
- Types of phosphorylation
References
- Oliveira, Ana Paula; Sauer, Uwe (2012-03-01). "The importance of post-translational modifications in regulating Saccharomyces cerevisiae metabolism". FEMS Yeast Research. 12 (2): 104–117. doi:10.1111/j.1567-1364.2011.00765.x. ISSN 1567-1364. PMID 22128902.
- Tripodi, Farida; Nicastro, Raffaele; Reghellin, Veronica; Coccetti, Paola (2015-04-01). "Post-translational modifications on yeast carbon metabolism: Regulatory mechanisms beyond transcriptional control". Biochimica et Biophysica Acta (BBA) - General Subjects. 1850 (4): 620–627. doi:10.1016/j.bbagen.2014.12.010. ISSN 0006-3002. PMID 25512067.
- Vlastaridis, Panayotis; Papakyriakou, Athanasios; Chaliotis, Anargyros; Stratikos, Efstratios; Oliver, Stephen G.; Amoutzias, Grigorios D. (2017-04-03). "The Pivotal Role of Protein Phosphorylation in the Control of Yeast Central Metabolism". G3 (Bethesda, Md.). 7 (4): 1239–1249. doi:10.1534/g3.116.037218. ISSN 2160-1836. PMC 5386872. PMID 28250014.
- Cohen, Philip (2002-05-01). "The origins of protein phosphorylation". Nature Cell Biology. 4 (5): E127–130. doi:10.1038/ncb0502-e127. ISSN 1465-7392. PMID 11988757.
- Vlastaridis, Panayotis; Kyriakidou, Pelagia; Chaliotis, Anargyros; de Peer, Yves Van; Oliver, Stephen G.; Amoutzias, Grigoris D. (2017-01-07). "Estimating the total number of phosphoproteins and phosphorylation sites in eukaryotic proteomes". GigaScience. 6 (2): 1–11. doi:10.1093/gigascience/giw015. ISSN 2047-217X. PMC 5466708. PMID 28327990.
- Walker DG, Rao S (1964). "The role of glucokinase in the phosphorylation of glucose by rat liver". Biochemical Journal. 90 (2): 360–8. doi:10.1042/bj0900360. PMC 1202625. PMID 5834248.
- Villar-Palasí, C.; Guinovart, J. J. (1 June 1997). "The role of glucose 6-phosphate in the control of glycogen synthase". The FASEB Journal. 11 (7): 544–558. doi:10.1096/fasebj.11.7.9212078. ISSN 0892-6638.
- Walker DG, Rao S (1964). "The role of glucokinase in the phosphorylation of glucose by rat liver". Biochemical Journal. 90 (2): 360–368. doi:10.1042/bj0900360. PMC 1202625. PMID 5834248.
- "Regulation of Glycolysis". cmgm.stanford.edu. Retrieved 2017-11-18.
- Sharma, Saumya; Guthrie, Patrick H.; Chan, Suzanne S.; Haq, Syed; Taegtmeyer, Heinrich (2007-10-01). "Glucose phosphorylation is required for insulin-dependent mTOR signalling in the heart". Cardiovascular Research. 76 (1): 71–80. doi:10.1016/j.cardiores.2007.05.004. ISSN 0008-6363. PMC 2257479. PMID 17553476.
- Chapter 14: Glycolysis and the Catabolism of Hexoses.
- Garrett, Reginald (1995). Biochemistry. Saunders College.
- "Hexokinase - Reaction". www.chem.uwec.edu. Retrieved 2020-07-29.
- Maber, Jon. "Introduction to Glycolysis". Retrieved 18 November 2017.
- Fuhs SR, Hunter T (2017). "pHisphorylation: the emergence of histidine phosphorylation as a reversible regulatory modification". Curr Opin Cell Biol. 45: 8–16. doi:10.1016/j.ceb.2016.12.010. PMC 5482761. PMID 28129587.
- Fuhs SR, Meisenhelder J, Aslanian A, Ma L, Zagorska A, Stankova M, Binnie A, Al-Obeidi F, Mauger J, Lemke G, Yates JR 3rd, Hunter T (2015). "Monoclonal 1- and 3-Phosphohistidine Antibodies: New Tools to Study Histidine Phosphorylation". Cell. 162 (1): 198–210. doi:10.1016/j.cell.2015.05.046. PMC 4491144. PMID 26140597.
- Hardman G, Perkins S, Brownridge PJ, Clarke CJ, Byrne DP, Campbell AE, Kalyuzhnyy A, Myall A, Eyers PA, Jones AR, Eyers CE (2019). "Strong anion exchange-mediated phosphoproteomics reveals extensive human non-canonical phosphorylation". EMBO J. 38 (21): e100847. doi:10.15252/embj.2018100847. PMC 6826212. PMID 31433507.
- Gonzalez-Sanchez MB, Lanucara F, Hardman GE, Eyers CE (2014). "Gas-phase intermolecular phosphate transfer within a phosphohistidine phosphopeptide dimer". Int J Mass Spectrom. 367: 28–34. Bibcode:2014IJMSp.367...28G. doi:10.1016/j.ijms.2014.04.015. PMC 4375673. PMID 25844054.
- Gonzalez-Sanchez MB, Lanucara F, Helm M, Eyers CE (2013). "Attempting to rewrite History: challenges with the analysis of histidine-phosphorylated peptides". Biochem Soc Trans. 41 (4): 1089–1095. doi:10.1042/bst20130072. PMID 23863184.
- Hardman G, Perkins S, Ruan Z, Kannan N, Brownridge P, Byrne DP, Eyers PA, Jones AR, Eyers CE (2017). "Extensive non-canonical phosphorylation in human cells revealed using strong-anion exchange-mediated phosphoproteomics". bioRxiv 10.1101/202820.