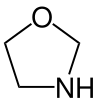
Oxazolidine
An oxazolidine is a five-membered ring compound consisting of three carbons, a nitrogen, and an oxygen. The oxygen and NH are the 1 and 3 positions, respectively. In oxazolidine derivatives, there is always a carbon between the oxygen and the nitrogen (or it would be an isoxazolidine).[1][2] All of the carbons in oxazolidines are reduced (compare to oxazole and oxazoline). Some of their derivatives, the oxazolidinediones, are used as anticonvulsants.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1,3-Oxazolidine | |
| Other names
Oxazolidine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.127.875 |
| MeSH | C064210 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H7NO | |
| Molar mass | 73.0938 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Oxazolidines were first synthesized over 100 years ago.[3]
Monooxazolidines
Oxazolidines that are the precursor to bisoxazolidines are in effect mono-oxazolidines. They are also used as moisture scavengers in Polyurethane and other systems. [4] [5]
Dioxooxazolidines
Oxazolidines where the carbon centers at the 1 and 3 positions are carbonyls are called dioxooxazolidines. Some of these are commercial fungicides including chlozolinate, vinclozolin, and famoxadone.[6]
Bisoxazolidines
Bisoxazolidines are chemical compounds that contain two oxazolidine rings, they are used as performance modifiers in polyurethane coatings and paints. The rings hydrolyze in the presence of moisture to give amine and hydroxyl groups, these can then react with diisocyanates, polyisocyanates and polyurethane prepolymers to form a coating.[7] The use of a bisoxazolidine in a polyurethane systems can prevent the unwanted reaction between isocyanate and moisture resulting in coating defects, as a result of carbon dioxide release. This moisture triggered curing route is preferential to moisture cure. As the ring opening reaction is catalyzed by acids, usually organic acids or anhydrides of carboxylic acids are added in a small amount.
The choice of linker between the two oxazolidine rings has a large impact on the performance when used to cure isocyanates. A rigid linker group increases a polyurethanes toughness. A flexible linker group imparts flexibility and increases elongation of a coating. These differences are the reason why bisoxazolidines are used to enhance the performance of polyurethane systems. Usually the rings are linked by esters, urethanes, carbonate or have the two rings fused together. A key intermediate in manufacturing bisoxazolidines is 2-[2-(propan-2-yl)-1,3-oxazolidin-3-yl]ethanol. The hydroxy group on the molecule allows for further reaction with hexamethylene diisocyanate for example.[8][9]
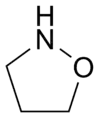
Isoxazolidines
In an isoxazolidine nitrogen and oxygen occupy are adjacent (positions 1 and 2 in the ring ), this is the saturated analogue of Isoxazole.
Isoxazolidines can be produced by the nitrone-olefin (3+2) cycloaddition reaction.
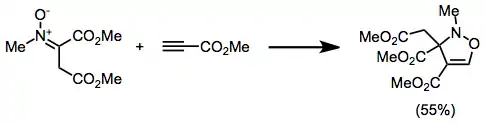
See also
- Imidazole, an analog with the oxygen replaced by a nitrogen.
- Thiazole, an analog with the oxygen replaced by a sulfur.
- Benzoxazole, where the oxazole is fused to another aromatic ring.
- Pyrrole, an analog without the oxygen atom.
- Furan, an analog without the nitrogen atom.
- Oxazoline, which has only one double bond reduced.
- Oxazolidinedione, which has two in-cycle keto groups (a carbamate and a lactam).
- Oxazolidinone, which has an in-cycle carbamate.
References
- "SID 3881507 -- PubChem Substance Summary". The PubChem Project. United States National Center for Biotechnology Information. Retrieved 13 December 2005.
- Dr Neil G Carter OXAZOLIDINE DILUENTS: REACTING FOR THE ENVIRONMENT Archived 2001-07-06 at the Wayback Machine Industrial Copolymers Limited
- Bergmann, Ernst D. (1953). "The Oxazolidines". Chemical Reviews. 53 (2): 309–352. doi:10.1021/cr60165a005.
- Florio, John J.; Miller, Daniel J. (2004-05-26). Handbook Of Coating Additives. CRC Press. ISBN 9780824756260.
- Howarth, Graham. "High Solids Polyurethane Coatings Using Oxazolidine Reactive Diluents" (PDF). Wikimedia commons.
- Franz Müller, Peter Ackermann, Paul Margot (2012). "Fungicides, Agricultural, 2. Individual Fungicides". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.o12_o06. ISBN 978-3527306732.CS1 maint: uses authors parameter (link)
- Emission control Archived 2003-01-06 at the Wayback Machine chembytes e-zine 2001.
- "2-[2-(propan-2-yl)-1,3-oxazolidin-3-yl]ethanol - Registration Dossier - ECHA". echa.europa.eu. Retrieved 2018-11-14.
- Howarth, GA (July 2003). "Polyurethanes, polyurethane dispersions and polyureas: Past, present and future". Surface Coatings International Part B: Coatings Transactions. 86 (2): 111–118. doi:10.1007/bf02699621. ISSN 1476-4865. S2CID 93574741.