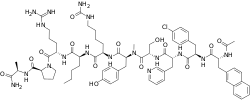
Ozarelix
Ozarelix (developmental code names D-63153, SPI-153) is a peptide gonadotropin-releasing hormone antagonist (GnRH antagonist) which is or was under development by AEterna Zentaris Inc. and Spectrum Pharmaceuticals as a long-acting injection formulation for the treatment of prostate cancer.[1][2] It has also been investigated for the treatment of endometriosis, but no development has been reported.[1] The drug was previously under investigation for the treatment of benign prostatic hyperplasia and Alzheimer's disease as well, but development for these indications was discontinued.[1] As of June 2015, it was in phase II clinical trials for prostate cancer.[1] It seems to no longer be under development.[1]
 | |
| Clinical data | |
|---|---|
| Other names | D-63153; SPI-153 |
| Routes of administration | Injection |
| Drug class | GnRH modulator; GnRH antagonist; Antigonadotropin |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.232.650 |
| Chemical and physical data | |
| Formula | C72H96ClN17O14 |
| Molar mass | 1459.12 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
See also
References
- http://adisinsight.springer.com/drugs/800013299
- Festuccia C, Dondi D, Piccolella M, Locatelli A, Gravina GL, Tombolini V, Motta M (2010). "Ozarelix, a fourth generation GnRH antagonist, induces apoptosis in hormone refractory androgen receptor negative prostate cancer cells modulating expression and activity of death receptors". Prostate. 70 (12): 1340–9. doi:10.1002/pros.21169. PMID 20623634. S2CID 2788093.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.